Sulfurized Two-Dimensional Conductive Metal–Organic Framework as a High-Performance Cathode Material for Rechargeable Mg Batteries
Abstract
Rechargeable Mg batteries are a promising energy storage technology to overcome the limitations inherent to Li ion batteries. A critical challenge in advancing Mg batteries is the lack of suitable cathode materials. In this work, we report a cathode design that incorporates S functionality into two-dimensional metal-organic-frameworks (2D-MOFs). This new cathode material enables good Mg2+ storage capacity and outstanding cyclability. It was found that upon the initial Mg2+ insertion and disinsertion, there is an apparent structural transformation that crumbles the layered 2D framework, leading to amorphization. The resulting material serves as the active material to host Mg2+ through reduction and/or oxidation of S and, to a limited extent, O. The reversible nature of S and O redox chemistry was confirmed by spectroscopic characterizations and validated by density functional calculations. Importantly, during the Mg2+ insertion and disinsertion process, the 2D nature of the framework was maintained, which plays a key role in enabling the high reversibility of the MOF cathode.
Recently, there has been a growing interest in developing rechargeable Mg batteries into a practical post-Li ion energy storage technology.1 Advantages offered by Mg batteries include the prospect of large-scale implementations owing to the abundance of Mg in the Earth's crust, the high theoretical volumetric capacities (up to 3,833 mAh/cm3), and the promise of safe operations.2 Nevertheless, rechargeable Mg batteries present several unique challenges, chief among which is the lack of suitable cathode materials to realize the purported high capacity.3 For instance, as a divalent cation, Mg2+ features high diffusion energy barriers in the lattice of most crystalline materials,4 making it difficult to achieve meaningful rechargeable performance through the intercalation mechanism.5 One way around this issue is to employ conversion-type cathodes that involve redox active compounds, such as S,6 O2,7 halogens,8 and organic materials.9 While stable redox behaviors and relatively fast kinetics are expected from these materials, they raise new concerns that are typical to implementations involving small molecules, including the shuttling effect, self-discharge, and difficulties in practical cell designs.10 Recognizing these challenges and opportunities, we show here that high-performance rechargeable Mg batteries as measured by capacities and rechargeability can be realized through a hybrid approach. Our strategy employs the two-dimensional metal–organic framework (2D-MOF) as a host material and introduces redox active S functional groups. The resulting materials enable desired Mg battery applications.
This work is inspired by prior successes of functionalization of MOF for Li−S battery applications.11 For example, it was reported by Thoi et al. that thiophosphate (PS42−) groups could be introduced to the metal nodes of Zr-MOFs, such as UiO-66 and MOF-808,12 which led to enhanced sulfur utilization and polysulfide encapsulation for delivering a sustainably high capacity over prolonged cycling. Separately, Kaskel et al. reported the postsynthetic sulfurization of dithiine-linked covalent organic frameworks (COFs), i.e., S-DUT-177, allowing for direct covalent attachment of polysulfides.13 Toward the same direction, there have been reports that suggest intercalation of cations, especially multivalent ones such as Mg2+, resulted in structural transformations of the host materials. For example, it was recently reported by Dincă et al. that 2D organic materials, e.g., bis-tetraaminobenzoquinone (BTABQ), exhibited pronounced interlayer expansion and a tendency of amorphization after electrochemical cycling in Li+-containing electrolyte.14 Taken as a whole, it becomes important to ask whether reversible Mg2+ intercalation is achievable using a framework host material with high charge storage capacities. It is within this context that we turn our attention to 2D-MOF because this class of materials features a layered structure that is ideal for ionic intercalation; the high electrical conductivity is an added benefit that is also desired for electrochemical energy storage applications.15 Indeed, 2D-MOF has already been studied as a new class of additives for Li−S battery applications, and promising results have been obtained.16 These progresses notwithstanding, key questions remain regarding the behaviors of 2D-MOF in electrochemical systems. For instance, relatively little attention has been paid to possible structural transformations of the framework upon intercalation (and deintercalation) of cations; while the amorphization of MOFs has been reported before,17 few studies focused on transformations due to electrochemical reactions in batteries. Most important of all, application of 2D-MOF in Mg-batteries have not been reported, to the best of our knowledge. We are, therefore, inspired to fill in the knowledge gap and report here our successes in improving Mg-battery performance with sulfurized 2D-MOF.
For this work, Cu-HHTP (HHTP=2,3,6,7,10,11-hexahydroxytriphenylene, Figure S7a) was chosen as a study platform to take advantage of the large body of existing literature on similar 2D-MOFs for electrochemical energy storage applications.18 New to our approach is the sulfurization strategy, which involved introducing S in the linker synthesis, followed by MOF preparation.
The sulfurized ligand, S5HHTP, was synthesized via a four-step molecular retrofitting as summarized in Scheme 1.19 Subsequently, Cu-S5HHTP was prepared following reported procedures (Figure 1a, see Supporting Information, SI, for details).20 The success of MOF preparation was indicated by the disappearance of the O−H stretching frequency in the Fourier transform infrared (FTIR) spectra at ca. 3300 cm−1 (Figure S8). It was observed in Figure 1b that Cu-S5HHTP and Cu-HHTP showed similar powder X-ray diffraction (PXRD) patterns. The introduction of S appeared to reduce the long-range order in the ab plane, which was evidenced by the reduced intensities of low angle diffraction peaks (e.g., those corresponding to (210) and (220) planes)21 in Cu-S5HHTP. Interestingly, the (001) diffraction peak in Cu-S5HHTP was more intense than Cu-HHTP, indicating an enhanced out-of-plane order due to the introduction of the S atoms. The results suggest that S atoms, due to its polarizability, could result in stronger van der Waals interactions than ligands without S, thereby enhancing weak intermolecular interactions between adjacent 2D layers. While increased π–π stacking interactions generally decrease interlayer distance, the (001) plane in Cu-S5HHTP shifted to a lower 2θ value in comparison to Cu-HHTP (27.36° vs 28.70°, respectively). This shift corresponds to an increased interlayer distance from 3.20 Å in Cu-HHTP to 3.40 Å in Cu-S5HHTP, presumably due to additional steric hindrance from waved S groups between neighboring layers. Such a structural distortion was further supported by scanning electron microscopy (SEM). As shown in Figure 1d, Cu-HHTP typically exhibited a rod-like morphology with a hexagonal cross-section and sub-micron in sizes. By comparison, Cu-S5HHTP showed a spherical morphology (Figure 1e), with no apparent resemblance of the hexagonal Kagome lattice observed in Cu-HHTP. Their electronic features were further evaluated by diffuse reflectance UV/Visible (DR UV/Vis) spectra (Figure 1c). Similar broad absorption bands stretching to the near-infrared (NIR) region was observed, suggesting that the linker conjugation is maintained upon ligand sulfurization.22 However, the slightly different spectral feature in the visible region revealed subtle differences in their electronic structures due to the ligand modification. These results imply that the conjugation of the ligand with sulfur potentially weakens the Cu−O interaction through S coordinating with Cu or modulating the charge density around oxygen.
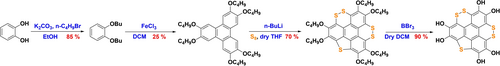
Synthesis of hexahydroxy-thienyl-triphenylene (S5HHTP).
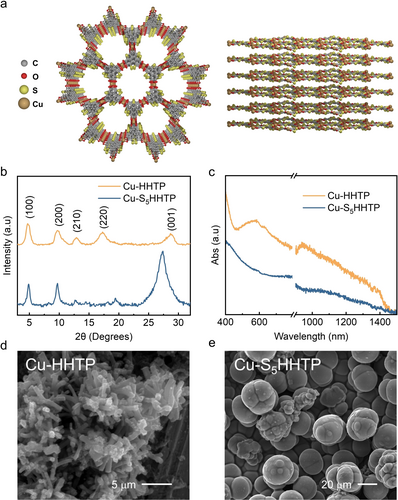
(a) Aligned stacking configuration of Cu-S5HHTP. (b) PXRD patterns of Cu-HHTP and Cu-S5HHTP. (c) DR UV/Vis spectra of Cu-HHTP and Cu-S5HHTP. (d, e) SEM images of Cu-HHTP (d) and Cu-S5HHTP (e).
Our next task was to study the behaviors of the different 2D-MOFs in Mg2+-based electrochemical systems.23 For this purpose, we fabricated cathodes with Cu-S5HHTP or Cu-HHTP as active materials in prototypical coin-type test cells (see Supporting Information for experimental details).24 Cycling performance was firstly recorded at a current density of 100 mA/g (based on the MOF mass; unless noted, all weights henceforth are relative to this reference) for 50 cycles. As shown in Figure 2a, an initial high capacity was measured on Cu-HHTP, which was not stable and quickly decayed to a stable baseline of ca. 300 mAh/g after 10 cycles. Similar features are frequently reported in the literature on comparable batteries,25 and it is often attributed to initial irreversible (but self-limiting) chemical reactions such as electrolyte and/or electrode decomposition that led to the formation of the electrolyte/electrode interphase layers.26 In comparison, the features for Cu-S5HHTP cathode were more complex. In addition to the initial rapid decay, down to ca. 300 mAh/g at the 4th cycle, a gradual recovery of the capacity was then observed, rising to a stable capacity of ca. 500 mAh/g at around the 15th cycle, followed by stable cycling for the rest of the test (see Supporting Information for additional discussion on the initial activation process). It has been reported that such capacity increase may arise due to the decrease of the particle size of the active materials or due to molecular contortion.27 To further study the effects of the initial cycles on the 2D MOF materials, we compared their XRD patterns before and after CV scans. It was found that the diffraction features corresponding to the π–π stacking by the 2D layers disappeared (Figure 2d). What remained were broad peaks that reported the loss of the original long-range ordered structure typical of the material, possibly representing an amorphous, contorted planar structure with Mg2+-bridged interlayer interactions.27b, 28 DR UV/Vis characterizations further confirmed that the coordination environment remained intact as evidenced by the strong absorption located at ca. 650 nm corresponding to the charge transfer between metal and ligand (Figure 2e).21 Taken as a whole, we understand that the 2D-MOF cathode materials have undergone structural transformations. Their long-range order was disrupted, but the 2D sheet structure was maintained and accompanied with crumpled features. This understanding was schematically depicted in the Graphical Abstract. Future research will likely be needed to fully understand the implications of this transformation on electrochemical behaviors. For the current study, we next focused on understanding the mechanisms by which the 2D-MOFs perform charge storage. Before moving on, however, we lastly note that the extra capacity (ca. 200 mAh/g) measured on Cu-S5HHTP in comparison to Cu-HHTP likely comes from the redox properties of the S functional groups in Cu-S5HHTP (see below).
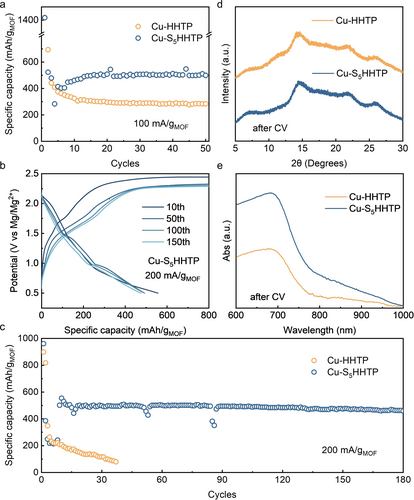
(a) Cycling performance of Cu-HHTP and Cu-S5HHTP at 100 mA/g. (b) Charge-discharge voltage profiles of Cu-S5HHTP at 200 mA/g. (c) Cycling stability of Cu-HHTP and Cu-S5HHTP at 200 mA/g. (d, e) Structural transformations in Cu-HHTP and Cu-S5HHTP following electrochemical cycles, as characterized by PXRD (d) and DR UV/Vis (e) measurements.
Broadly speaking, three types of contribution could be used to account for the measured capacity in Cu-S5HHTP, namely the capacitive charge storage owing to the high surface area of the active materials (Figure S9), the redox properties of the HHTP ligand, and the extra redox activities from the S functional groups. If we assume each S in a redox unit stores one electron, an extra capacity of ca. 230 mAh/g would be expected from Cu-S5HHTP (see Supporting Information for more details on the capacity calculations). As such, the measured 200 mAh/g extra capacity is reasonable and implies the average charge stored on every S is approximately 1. We further measured the cycling stability of Cu-S5HHTP cathode at 200 mA/g for 180 cycles. During the cycling, we observed that the discharge capacity of Cu-S5HHTP cathode reached a steady-state capacity of ca. 500 mAh/g after 10 cycles (Figure 2b, see Supporting Information for additional discussion on the overcharging effect), corresponding to ca. 580 Wh/kg specific energy density and an average working potential of 1.1 V (vs. Mg/Mg2+; unless noted, all potentials henceforth are relative to this reference). The capacity slightly decreased to ca. 460 mAh/g towards the end, illustrating that the runaway of active materials is significantly suppressed (Figure 2c). In a typical Mg−S system, only 7 % of the peak capacity (ca. 60 mAh/g) can be retained after 70 cycles,29 largely due to the polysulfide shuttling effect that causes the loss of active materials and the passivation of Mg anode surface.30 Cu-S5HHTP effectively addressed these detrimental effects, promising a new type of cathode material that takes advantage of both 2D-MOF and S redox chemistries. Beyond this, by comparison with Cu-HHTP, it is worth noting that covalently attached S can potentially stabilize the HHTP-based MOFs under high current density. Fast capacity decay was recorded for Cu-HHTP cathode at 200 mA/g, suggesting the irreversible disintegration of the redox framework as current densities increased (see below).
Next, our objective is to understand how Cu-S5HHTP stores Mg2+ (see Supporting Information for additional discussion on the potential role of MgCl+ for energy storage) under electrochemical conditions. Computational results indicated that the most stable Mg binding configurations correspond to sites where Mg can simultaneously interact with S atoms of the MOF linker and with O atoms of the [CuO4] node (Figure 3a, see Supporting Information for additional discussion on the computational study and results). This understanding was further validated by monitoring the shifts in the chemical environment of O and S elements across different discharge states, which was achieved through ex situ X-ray photoelectron spectroscopy (XPS) measurements (Figure 3b). O 1s spectra displayed a clear reversible interconversion between quinoid (electron-deficient, ca. 532 eV) and benzoid (electron-rich, ca. 533 eV) features in response to changes in potentials (Figure S12b).18b In contrast, the O chemistry of Cu-HHTP exhibited poor reversibility, with lower quinoid feature recovered after recharging (Figure S11a), implying the potential deactivation of Cu-HHTP during electrochemical cycling. Furthermore, the analysis of S 2p spectra revealed that the S species in Cu-HHTP cathodes originated exclusively from the adsorption and decomposition of TFSI anions during the electrochemical processes (Figure S11b). By comparison, Cu-S5HHTP cathodes demonstrated S−S and M−S (where M represents metal) interactions across all discharge and charge states,31 in which the intensity of M−S state reversibly changed. Additionally, significant adsorption of TSFI was observed in the charged state of Cu-S5HHTP cathode, suggesting its behavior as a bipolar-type cathode.9c The computational and XPS results together suggested that the presence of S atoms mediates Mg interaction with the [CuO4] node, expanding the number of available Mg binding sites and increasing the capacity of the 2D-MOF cathode (Figure S14c). It is reasonable to presume that the reduction of the [CuO4] coordination complex may lead to the weakening of the binding strength between the linker and the metal node, which would compromise structural integrity, resulting in potentially irreversible disintegration. Such a problem would be especially acute for a high degree of discharge when more reduction is expected. The challenge may be addressed by the sulfurization strategy because the reversible redox activity of the S functional groups would minimize irreversible changes to the node complexes. Computational evdence suggests that the simultaneous binding of Mg to S and O atoms stabilizes the [CuO4] node by drawing electron density away from O onto S (see SI), in support of this explanation. Future efforts should focus on identifying conditions that favor desirable redox reactions while minimizing undesired side reactions.
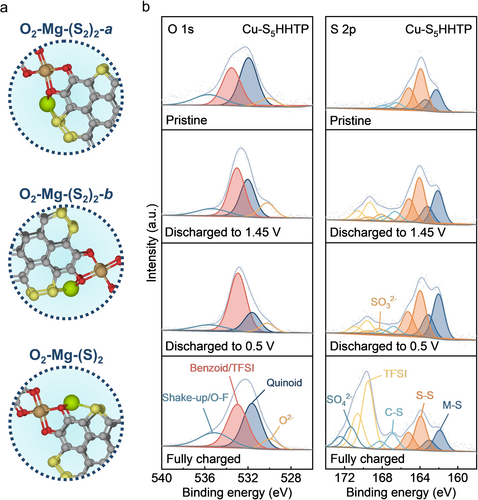
(a) Density functional theory (DFT)-optimized binding configurations of Mg in Cu-S5HHTP through synergistic O and S redox chemistries. (b) Ex situ high-resolution O 1s and S 2p XPS of Cu-S5HHTP during discharge-charge.
More insights into charge storage were further collected through Dunn's method,32 a commonly employed approach to distinguish between charge-storage contributions originating from capacitance and those resulting from bulk ion diffusion. In a typical experiment, we conducted cyclic voltammetry measurements with varying scan rates from 0.1 to 10 mV/s (Figure 4a and Figure S17). Our results indicated that ca. 60 % of the measured capacity in Cu-S5HHTP cathode can be ascribed to the capacitive effect at high scan rates (>5 mV/s, Figure 4b), whereas at lower scan rates, the processes limited by bulk ion diffusion account for a relatively major contribution to the overall measured capacity (e.g., ca. 90 % at 0.1 mV/s) (Figure 4c). These findings suggest that the cathode benefits from a high capacitive contribution at rapid scan rates due to its conjugated basal plane and the plentiful surface redox-active sites. It was further observed in Figure 4d that introducing S functionality enhanced the capacitive storage during discharge. The role of bulk ion diffusion became more pronounced for the charge storage in Cu-HHTP at lower potentials, where the depth of discharge is increased. These results suggest that upon deep discharge, the charge storage processes limited by bulk ion diffusion are more likely to lead to the disintegration of the framework irrespective of the sulfurization.
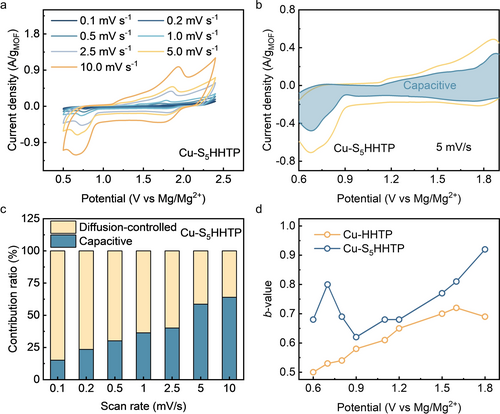
(a) CV curves of Cu-S5HHTP recorded at various scan rates. (b) Capacitive current (the blue part) contributed to the overall charge-storage of Cu-S5HHTP at the rate of 5 mV/s. (c) Capacitive contributions at various scan rates. (d) b-values for Cu-HHTP and Cu-S5HHTP plotted as a function of the potential for cathodic scans.
In summary, we have demonstrated a new strategy in the development of high-performance cathode materials for rechargeable Mg batteries, achieved by covalently anchoring S into 2D-MOFs. The proof-of-concept material, Cu-S5HHTP, featured high capacity (ca. 500 mAh/g) and remarkable cyclability (retaining ca. 460 mAh/g after 180 cycles). Through mechanistic investigations, we uncovered the redox behavior of S within the framework, which plays a significant role in minimizing the disintegration of the MOF basal plane upon structural transformations. This insight paves the way for rationalizing the application of 2D-MOFs in electrochemical energy storage by using Mg.
Supporting Information
Experimental procedures, additional figures, and discussions.
Acknowledgments
This work is supported by the National Science Foundation through project DMR 2126923. Y.M. acknowledges the support from Chia-Kuang (Frank) Tsung Graduate Fellowship at Boston College. J. N. acknowledges the support from O'Brien Fellowship at Marquette University.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




