First Main-Group Element Lewis Acid Thionyl Chloride Adduct and its Chemistry
Abstract
Although the adduct of aluminum trichloride with thionyl chloride has been reported, no thionyl chloride adduct of a main group element Lewis acid or organometallic compound has been structurally characterized. In this communication we present the synthesis and reactivity of the structurally ascertained adduct of thionyl chloride with tris(pentafluoroethyl)gallane as a representative of a main group element Lewis acid. Gallium and indium compounds with electron withdrawing groups, e.g. the pentafluoroethyl ligand, display versatile properties. While gallates and indates, [MR4]−, behave as weakly coordinating anions, neutral gallanes and indanes, MR3, are strong Lewis acids. Salts with the tetrakis(pentafluoroethyl)gallate and -indate, [M(C2F5)4]− (M=Ga, In), have recently been studied in detail. In contrast to this, work on the syntheses of the free Lewis superacids M(C2F5)3 (M=Ga, In) is scarce and underdeveloped. The hydrates [M(C2F5)3(OH2)2] proved to be suitable starting materials, particularly due to their thermal stability. Herein we report on synthesis and characterization of reactive adducts, [M(C2F5)3D], with the weak donor molecules (D) SOCl2 and Me3SiF. The effective Lewis acidities of Ga(C2F5)3 and In(C2F5)3 were experimentally determined by the (modified) Gutmann-Beckett method and their catalytic potential is showcased.
Introduction
Elements of the main groups III and V are the most prominent central atoms for Lewis acids. Strong Lewis acids with a central atom of main group V are usually based on bismuth and antimony as well as phosphorus, whereas Lewis acids with elements of main group III are mainly represented by boron and aluminum.1 While the Lewis acidity of the main group elements of group V increases towards the heavier analogs, a reverse trend is evident in main group III.1, 2
In addition, strong Lewis acids with elements of main group IV, silicon and germanium, have been recently reported.3-5 Selected examples are shown in Figure 1.
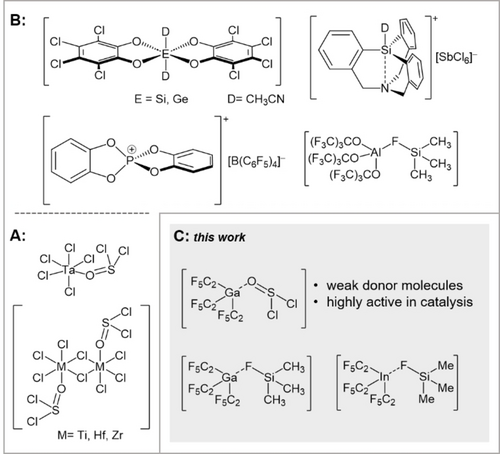
Lewis acids can be classified in various ways. The global calculated Lewis acidity takes the entire process into account and provides thermodynamic values.6 The most commonly used scale is the fluoride ion affinity (FIA). The FIA is defined as the negative enthalpy of the gas phase reaction between a fluoride ion and a Lewis acid and reflects the hardness of a Lewis acid. Lewis acids that exceed the FIA of SbF5 are classified as hard Lewis superacids.7 The hydride ion affinity (HIA), on the other hand, mirrors the softness of a Lewis acid. Here B(C6F5)3 is used as a benchmark compound and all Lewis acids with a higher HIA are categorized as soft Lewis superacids.2, 6-8
In contrast, the effective Lewis acidity describes the direct interaction of a Lewis acid with a donor molecule by spectroscopic methods (e.g. NMR spectroscopy). Here, the most popular procedure is the Gutmann-Beckett method utilizing the induced phosphorus chemical shift of triethylphosphine oxide caused by Lewis acid coordination.2 One way to estimate the softness of a Lewis acid is the modified Gutmann-Beckett method, in which the trimethylphosphine sulfide adduct is formed. Analogously to the original Gutmann-Beckett method the phosphorus NMR shift is considered.9
The reactivity of a Lewis acid can be strongly suppressed by strong donor molecules. As thionyl chloride is a very weak electron donor,15 high reactivity of corresponding complexes can be expected. While only a few examples of structurally characterized transition metal chloride adducts of thionyl chloride are known in literature (Figure 1A), adducts of thionyl chloride with Lewis acids based on main group elements have not been structurally characterized.10-12 While the zirconium and hafnium chlorides form complexes of the composition [MCl4(SOCl2)]2, which are even stable up to at least 373 K, titanium forms a complex of the same composition which decomposes above 220 K. The tantalum derivative of the composition [TaCl5(SOCl2)] is thermally unstable above 290 K.10-12
Lewis superacids based on gallium and indium are rare. Gallium and indium compounds are less Lewis acidic than their aluminum analogs, due to higher electronegativity and d-block contraction.1, 2 Recently we published the synthesis of the soft Lewis superacid tris(pentafluoroethyl)indane as its dihydrate, [In(C2F5)3(OH2)2], and an improved synthesis for the hard and soft Lewis superacidic gallium analog.16
Herein we present the synthesis of tris(pentafluoroethyl)gallium and -indium adducts with weak donor molecules. Additionally, an outlook on anticipated catalytic capabilities of these Lewis superacids is given in this report.
Results and Discussion
Weak Donor Adducts of M(C2F5)3 (M=Ga, In)–Synthesis, Characterization and Chemistry
Recently, we published that the treatment of a dichloromethane solution of [M(C2F5)3(OH2)2] (M=Ga, In) with molecular sieves (3 Å) does not lead to the complete removal of water, but to the formation of a hydroxide-bridged gallate and indate, K[(F5C2)3M(μ-OH)M(C2F5)3].16 In a further effort to remove the water from tris(pentafluoroethyl)gallane and -indane dihydrate we reacted them with an excess of thionyl chloride. Subsequent removal of all volatile compounds yielded highly reactive residues (Scheme 1). In the case of gallium as the central atom crystals suitable for X-ray diffraction could be obtained by in situ crystallization in a capillary (Figure 2). [Ga(C2F5)3(SOCl2)] is the first structurally elucidated thionyl chloride adduct of a main group element Lewis acid. [Ga(C2F5)3(SOCl2)] crystallizes in the orthorhombic space group P212121 with four formula units per unit cell. The sulfur atom is hexacoordinated with intermolecular contacts to fluorine atoms (F1, F7 and F14) of three other gallanes. Hexacoordination can also be found, for example, in the molecular structure of free SOCl2, and the tantalum complex, [TaCl5(SOCl2)].11, 17 The O−S bond (1.4777(16) Å) is slightly elongated in comparison to non-coordinated SOCl2 (1.4394(10) Å).17 The sum of the Cl−S−Cl and O−S−Cl angles (311.7°) is comparable to that in uncoordinated SOCl2 (311.1°).17 The Ga−C bond lengths of 2.016(2)–2.020(2) Å are comparable to those in other pentafluoroethyl gallium compounds.18, 19 The sum of the C−Ga−C bond angles (350.2°) is comparable with that in the literature-known gallane adduct [Ga(C2F5)3{(OH2)(OEt2)}].18
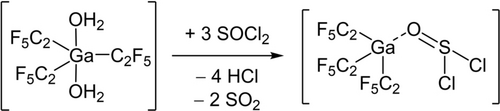
Synthesis of [Ga(C2F5)3(SOCl2)] by the reaction of [Ga(C2F5)3(OH2)2] with SOCl2.
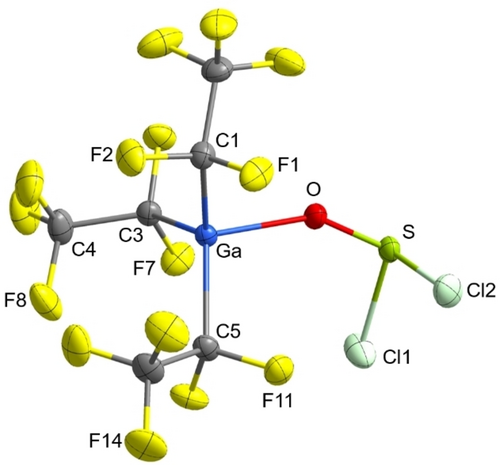
Molecular structure of [Ga(C2F5)3(SOCl2)]. Thermal ellipsoids are set at 50 % probability. Selected bond lengths [Å] and angles [°]: Ga−O 1.9950(15), O−S 1.4777(16), S−Cl1 2.0055(8), S−Cl2 1.9990(8), Ga−C1 2.016(2), Ga1−C3 2.017(2), Ga1−C5 2.020(2), C1−F1 1.374(3), C1−F2 1.371(3), Ga−O−S 147.91(11), C1−Ga1−C3 119.15(9), C3−Ga1−C5 116.07(9), C5−Ga1−C1 114.93(9).
When the thionyl chloride adducts were dissolved in an excess of fluorotrimethylsilane, the SOCl2 is substituted by Me3SiF.
After removal of all volatiles [M(C2F5)3(FSiMe3)] (M=Ga, In) is obtained (Scheme 2).
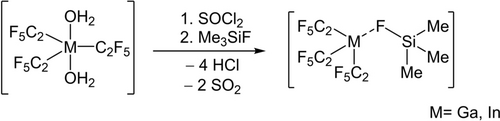
Synthesis of [M(C2F5)3(FSiMe3)] by the reaction of [M(C2F5)3(OH2)2] (M=Ga, In) with SOCl2 followed by Me3SiF.
In the 19F NMR spectra the resonances of the fluorotrimethylsilane unit compared to pure Me3SiF are shifted from −157.8 ppm to −176.2 ppm for [Ga(C2F5)3(FSiMe3)] and −167.4 ppm for [In(C2F5)3(FSiMe3)] and are significantly broadened. 1H/29Si-HMBC NMR measurements allowed determining the 29Si chemical shifts at 58.9 ppm ([Ga(C2F5)3(FSiMe3)]) and 55.4 ppm ([In(C2F5)3(FSiMe3)]), which represents a significant downfield shift compared to the 33.2 ppm of non-coordinated Me3SiF. The upfield shift and the broad lines in the 19F NMR spectrum, as well as the 29Si NMR shift indicate a coordination of the fluorine of Me3SiF to the gallium and indium atoms. The elemental analysis confirms a composition with only one fluorotrimethylsilane as donor. This implies tetracoordination for the gallium and indium atoms.
[Ga(C2F5)3(FSiMe3)] is a colorless liquid with a vapor pressure of less than one millibar. [In(C2F5)3(FSiMe3)] was obtained as a colorless, crystalline and very hygroscopic solid after careful sublimation. The sublimed product contained crystals suitable for X-ray diffraction analysis. In the case of gallium as central atom crystals suitable for X-ray diffraction could be obtained via in situ crystallization in a capillary. X-ray diffraction analysis of both fluorosilane adducts revealed dimeric structures with one fluorotrimethylsilane ligand coordinated to the Ga(C2F5)3 and In(C2F5)3 moieties and with bridging contacts via the α-fluorine atoms of two pentafluoroethyl groups to the gallium and indium atoms (Figure 3 and 4).
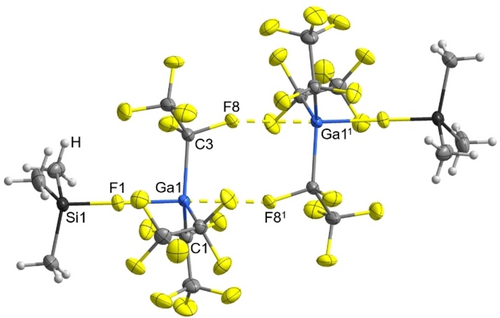
Molecular structure of [Ga(C2F5)3(FSiMe3)]2. Thermal ellipsoids are set at 50 % probability. Selected bond lengths [Å] and angles [°]: Ga1−F1 2.0240(6), Si1−F1 1.6958(7), Ga11⋅⋅⋅F8 2.5871(7), Ga1−C1 2.0185(11), Ga1−C3 2.0219(11), Ga1−C5 2.0200(11), C3−F8 1.3915(12), C3−F7 1.3680(13), F81−Ga1−F1 177.59(2), Si1−F1−Ga1 164.69(4), C3−Ga1−F1 95.65(4), C1−Ga1−C3 113.34(5), C3−Ga1−C5 119.91(5), C5−Ga1−C1 122.90(5). Symmetry code: 1-x,1-y,1-z
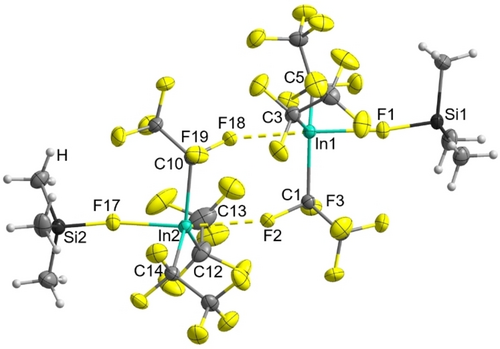
Molecular structure of [In(C2F5)3(FSiMe3)]2. The CF3 group at C13 is disordered over two sites with a ratio of 83 : 17. Only the major occupied atoms are shown for clarity. Thermal ellipsoids are set at 50 % probability. Selected bond lengths [Å] and angles [°]: In1−F1 2.244(2), In2−F17 2.252(2), Si1−F1 1.679(2), Si2−F17 1.672(2), In1⋅⋅⋅F18 2.563(2), In2⋅⋅⋅F2 2.595(2), In1−C1 2.221(4), In1−C3 2.206(4), In1−C5 2.209(3), In2−C10 2.210(4), In2−C12 2.199(4), In2−C14 2.202(4), C1−F2 1.401(4), C1−F3 1.357(4), C10−F18 1.408(4), C10−F19 1.362(4), F1−In1−F18 167.5(1), F2−In2−F17 172.10(8), Si1−F1−In1 166.2(1), In2−F17−Si2 166.5(1), C5−In1−F1 91.0(1), C1−In1−C3 118.1(1), C3−In1−C5 126.5(1), C5−In1−C1 113.9(1), C10−In2−C12 125.5(2), C12−In2−C14 118.8(2), C14−In2−C10 114.9(1).
Due to the contacts to the C2F5 groups the gallium and indium atoms attain the geometry of a trigonal-bipyramid as it is common for many gallanes and indanes like the hydrates or donor complexes of In(C6F5)3.18, 20 The coordination of Me3SiF to the Lewis acids Ga(C2F5)3 and In(C2F5)3 leads to an increase in the Si−F bond length from 1.600(2) Å in free Me3SiF to 1.6958(7) Å in its adduct with Ga(C2F5)3 and to an average of 1.673 Å with In(C2F5)3.21 The sum of the C−Si-C bond angles allows a classification of the ionic character of the silane. A covalent Me3Si−Y has a sum of bond angles of 329° while a purely ionic [Me3Si]+ Y− has a sum of 360°. The continuum between 345–354° can be viewed as ion-like.14 In the case of [Ga(C2F5)3(FSiMe3)] the sum of the C−Si−C bond angles is 343° and in [In(C2F5)3(FSiMe3)] in average 343°, supporting a more covalently bound Me3Si−F in both cases.
A more pronounced interaction was observed in the adduct of Me3SiF to the hard Lewis superacid Al[OC(CF3)3]3.14 [Al{OC(CF3)3}3(FSiMe3)] has a sum of C−Si−C bond angles of 346° and an elongation of the Si−F distance of 0.05 Å in comparison to [Ga(C2F5)3(FSiMe3)] and 0.07 Å to [In(C2F5)3(FSiMe3)]. This hard-hard interaction between the aluminum Lewis acid and Me3SiF is also reflected in the 29Si NMR shift of 82.3 ppm.14 The analysis of the C−Si−C bond angles and the 29Si NMR shift of 59.8 pm in [Ga(C2F5)3(FSiMe3)] and 55.4 ppm in [In(C2F5)3(FSiMe3)] affirms in both cases the hard-soft interaction between the fluorine and the gallium respectively the indium atoms.
These differences in structural parameters and spectroscopic data also describe the differences in reactivity. [Ga(C2F5)3(FSiMe3)] reacts with trimethylphosphine to give the corresponding phosphine adduct (Scheme 3). When the hard Lewis acid [Al{OC(CF3)3}3(FSiMe3)] is treated with a phosphine, a silyl phosphonium cation is obtained.14
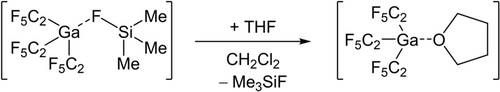
Synthesis of [Ga(C2F5)3(thf)] by the reaction of [Ga(C2F5)3(FSiMe3)] with tetrahydrofuran.
In case of alcohols as hard Lewis bases, ligand exchange is observed for [Ga(C2F5)3(FSiMe3)] and [Al{OC(CF3)3}3(FSiMe3)].14
Syntheses of [Ga(C2F5)3(PMe3)] and [Ga(C2F5)3(i-PrOH)] are straightforward, affording the products in good yields, which allow X-Ray structural analysis (Figure 5).
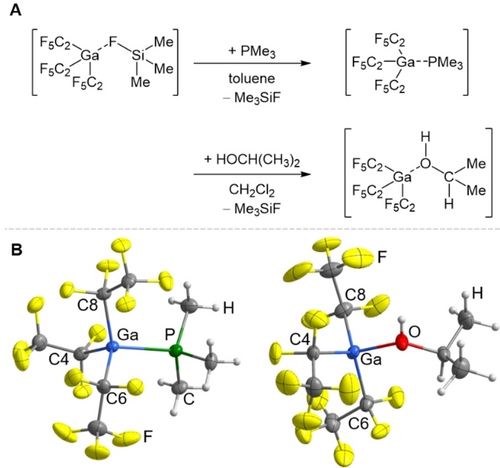
A: Synthesis of [Ga(C2F5)3(PMe3)] and [Ga(C2F5)3{HOCH(CH3)2}] by the reaction of [Ga(C2F5)3(FSiMe3)] with trimethylphosphine and isopropyl alcohol. B: Molecular structure of [Ga(C2F5)3(PMe3)] and [Ga(C2F5)3(HOCH(CH3)3]. Thermal ellipsoids are set at 50 % probability; minor occupied disordered atoms are omitted. Selected bond lengths [Å] and angles [°] for [Ga(C2F5)2(PMe3)]: Ga1−P1 2.3682(11), Ga1−C8 2.032(4), Ga1−C4 2.032(4), Ga1−C6 2.028(4), P1−C1 1.806(4), C4−Ga1−C6 110.88(16), C4−Ga1−C8 115.78(17), C6−Ga1−C8 108.71(15). Selected bond lengths [Å] and angles [°] for [Ga(C2F5)2(i-PrOH)]: Ga−O 1.9549(11), Ga−C4 2.0242(15), Ga−C6 2.0214(15), Ga−C8 2.051(3), C4−Ga−C6 119.35(6), C4−Ga−C8 113.19(9), C6−Ga−C8 120.46(11).
Effective Lewis Acidities
Having in hand tris(pentafluoroethyl)gallane and -indane in form of their adducts to the weak Lewis basic donor fluorotrimethylsilane, it was possible to experimentally assess their Lewis acidity. The commonly applied Gutmann-Beckett test uses Et3PO as a 31P NMR probe and the shift after complexation to the Lewis acid is measured.
While the reaction between triethylphosphine oxide and tris(pentafluoroethyl)indane leads to [In(C2F5)3(OPEt3)], which can be observed by NMR spectroscopy, tris(pentafluoroethyl)gallane fluorotrimethylsilane reacts with triethylphosphine oxide leading to a product mixture due to the high reactivity of the gallane adduct. Therefore, it is necessary to replace the fluorosilane by tetrahydrofuran (THF) (Scheme 3). [Ga(C2F5)3(thf)] reacts with Et3PO in a ligand exchange reaction leading to the triethylphosphine oxide adduct.
The addition of [Ga(C2F5)3(thf)] to Et3PO led to a shift of Δδ(31P)=29.0 ppm, while [In(C2F5)3(Me3SiF)] gave a shift of 24.4 ppm and [In(C2F5)3(thf)] a shift of 22.5 ppm. Addition of B(C6F5)3 results in a shift of Δδ(31P)=26.6 ppm (Figure 6).22, 23 In line with the global calculated Lewis acidity, Ga(C2F5)3 is a stronger Lewis acid than B(C6F5)3. Contrary to expectations from the calculated Lewis acidities (Table 1), In(C2F5)3 has the smaller shift. This finding would identify In(C2F5)3 as the weaker Lewis acid in comparison to B(C6F5)3.
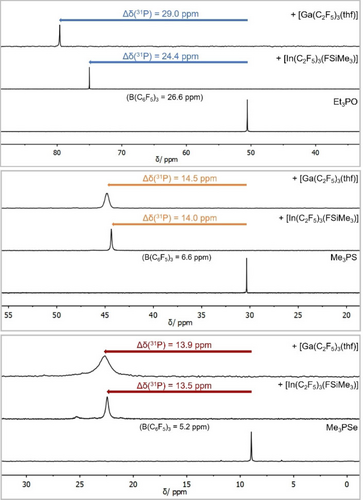
Comparison of the Gutmann-Beckett method with its modified version. 31P{1H} NMR spectra of the corresponding Lewis bases were measured in CD2Cl2 and their relative shifts after addition of [Ga(C2F5)3(thf)] and [In(C2F5)3(FSiMe3)] were determined. The reported relative shifts after addition of B(C6F5)3 were taken as references.9, 22, 23
Keeping in mind that Et3PO is considered a hard Lewis base according to the Pearson concept, the modified Gutmann-Beckett test was employed.9, 24 The modified method uses the 31P NMR shifts of Me3PS and Me3PSe and should be more meaningful for the assessment of soft Lewis acids. By adding [Ga(C2F5)3(thf)] to Me3PS the signal shifts by Δδ(31P)=14.5 ppm in comparison to free Me3PS. [In(C2F5)3(FSiMe3)] gave a shift of 14.0 ppm and [In(C2F5)3(thf)] a shift of 11.4 ppm. In case of Me3PSe a relative shift of Δδ(31P)=13.9 ppm for [Ga(C2F5)3(thf)], 13.5 ppm for [In(C2F5)3(FSiMe3)] and 13.0 ppm for [In(C2F5)3(thf)] is observed. In all cases the shifts are significantly stronger than with B(C6F5)3 (Δδ(31P)=6.6 ppm for Me3PS, Δδ(31P)=5.2 ppm for Me3PSe, Figure 6).9 The use of the THF adduct of tris(pentafluoroethyl)indane in the (modified) Gutmann-Beckett method results in minimally smaller, but nevertheless comparable shifts with respect to the use of the fluorosilane adduct. These experimental results are supported by calculations of the global Lewis acidity and the classification of Ga(C2F5)3 and In(C2F5)3 as soft Lewis superacids.7, 8, 25 The significant difference in case of In(C2F5)3 between the classic Gutmann-Beckett method and its modified version demonstrates how crucial the assessment of the hard and soft nature of the employed Lewis base for reliable results is.
Single crystals of [Ga(C2F5)3(SPMe3)] suitable for X-ray diffraction were grown from a dichloromethane solution. [Ga(C2F5)3(SPMe3)] crystallizes in the orthorhombic space group Pbcn with eight formula units per unit cell (Figure 7). As expected the P−S bond (2.0268(5) Å) is significantly elongated compared to the P−S bond in uncoordinated Me3PS (1.97 Å)9 and comparable to the distance in the cationic Lewis superacid adduct [Bi(diaryl)(SPMe3)][SbF6].9
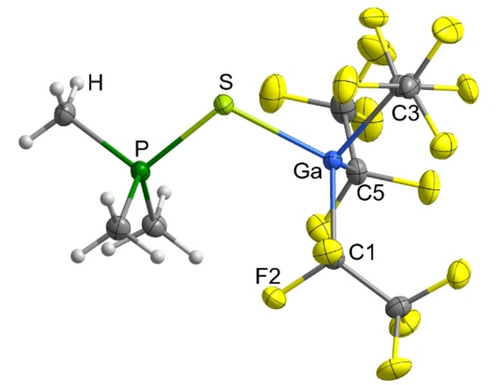
Molecular structure of [Ga(C2F5)3(SPMe3)]. Thermal ellipsoids are set at 50 % probability. Selected bond lengths [Å] and angles [°]: Ga−S 2.2954(4), Ga−C1 2.0301(14), Ga−C3 2.0344(14), Ga−C5 2.0323(14), S−P 2.0268(5), P−C7 1.7868(15), P−C8 1.7877(15), P−C9 1.7878(15), C1−F2 1.3767(16), P−S−Ga 111.057(19), C1−Ga1−C3 115.14(6), C3−Ga1−C5 109.56(6), C5−Ga1−C1 109.84(6).
[M(C2F5)3(FSiMe3)] (M=Ga, In)—Catalytic Capabilities
The large interest in Lewis acids is based on their catalytic potential. Therefore, after identifying Ga(C2F5)3 as a hard and soft and In(C2F5)3 as a soft Lewis superacid, we tested their promising potential as a catalyst with the transformations depicted in Scheme 4.
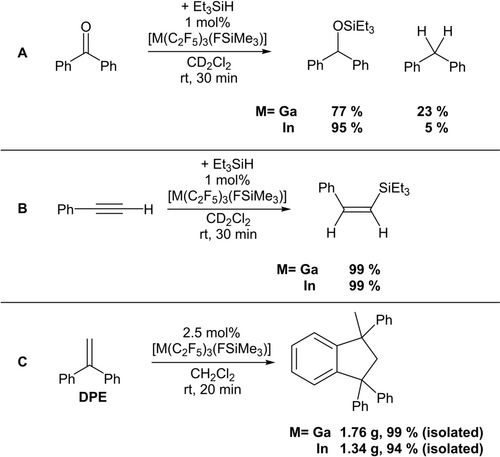
Catalytic reactions with [M(C2F5)3(FSiMe3)] (M=Ga, In). A: Hydrosilylation of benzophenone, B: Hydrosilylation of phenylacetylene, C: Catalytic Friedel–Crafts dimerization of 1,1-diphenylethylene.
Classic Lewis-acid catalyzed reactions like the hydrosilylation of carbonyls or alkynes proceed smoothly with low catalyst loadings of [M(C2F5)3(FSiMe3)] (M=Ga, In) at room temperature. In case of [In(C2F5)3(FSiMe3)] the hydrosilylation of benzophenone yielded the silyl ether as the major product while also minor quantities of the deoxygenation product diphenylmethane were formed. In case of [Ga(C2F5)3(FSiMe3)] the silyl ether was formed at 77 % and the deoxygenation product at 23 %. As expected for a Lewis acid catalyzed hydrosilylation of alkynes, the (Z)-addition product was exclusively obtained and only traces of the α-product were found. Furthermore, 1,1-diphenylethylene (DPE) reacts with a catalytic amount of [M(C2F5)3(FSiMe3)] (M=Ga, In)] in a Friedel–Crafts dimerization. Full conversion was already observed after 20 minutes at room temperature. Repetition of this experiment on a larger scale emphasizes the practicability of this reaction and the dimerization product of DPE could be obtained as a colorless, crystalline solid in a yield of 1.76 g (99 %) (Ga(C2F5)3) and 1.34 g (94 %) (In(C2F5)3) (Scheme 4).
As already mentioned the tris(pentafluoroethyl)gallane is a hard and soft Lewis superacid whereas tris(pentafluoroethyl)indane is a soft Lewis superacid by definition and also shown via the (modified) Gutmann-Beckett method. However, the calculated FIA and HIA of tris(pentafluoroethyl)gallane and -indane are relatively low compared to those of current literature examples, especially compared to cationic Lewis acids (Table 1).
However, these values correspond to the donor-free Lewis acids, not to the donor adducts which are actually synthesized. As previously mentioned, tris(pentafluoroethyl)gallane and also the indane are accessible as adducts with the weak fluorosilane donor. The germanium catecholate Ge(catCl)2 and the atrane type silane [Si(atrane)]+, on the other hand, were synthesized with the stronger donor acetonitrile (Figure 1).3, 4 Only the catecholato phosphonium Lewis acid [P(catH)2]+ was synthesized free from donor molecules (Figure 1).13
When the germanium catecholate and the silicon atrane-type Lewis acids with high ion affinities, but strong donor molecules, were used in a Friedel–Crafts dimerization of 1,1-diphenylethylene, reflux conditions and long reaction times were required. Only the catecholato-phosphonium Lewis acid exhibits a comparably rapid conversion as Ga(C2F5)3 and In(C2F5)3 at room temperature. Remarkably, the reactivity of Ga(C2F5)3 and In(C2F5)3, despite significantly lower ion affinities, is comparable or even superior to the mentioned Lewis superacids.
Conclusion
In this work the synthesis of weak donor adducts of the Lewis superacids Ga(C2F5)3 and In(C2F5)3 is reported. Adducts are accessible by dehydration of [M(C2F5)3(OH2)2] (M=Ga, In) with thionyl chloride. In the case of gallium as central atom, [Ga(C2F5)3(SOCl2)] was isolated and characterized by NMR spectroscopy and X-ray diffraction. [Ga(C2F5)3(SOCl2)] is the first structurally characterized thionyl chloride adduct of a main group element Lewis acid. By dissolving the thionyl chloride adducts in fluorotrimethylsilane, SOCl2 was replaced by Me3SiF. [M(C2F5)3(FSiMe3)] (M=Ga, In) was characterized by NMR spectroscopy, X-ray diffraction, and elemental analysis. [Ga(C2F5)3(PMe3)] and [Ga(C2F5)3{HOCH(CH3)2}] were obtained by substitution reactions. The effective Lewis acidity was determined by the (modified) Gutmann-Beckett method, which confirmed the hard and soft Lewis superacidic character of Ga(C2F5)3 and the soft Lewis superacidic character of In(C2F5)3.
The catalytic potential of [M(C2F5)3(FSiMe3)] (M=Ga, In) was investigated in several test reactions, including hydrosilylation of carbonyls and alkynes as well as the dimerization of 1,1-diphenylethylene. Equivalent or even superior reactivity was found in comparison to other Lewis superacids. The water tolerance of Ga(C2F5)3 and In(C2F5)3 provides a perspective for Lewis superacid catalysis in water.
Acknowledgments
We are grateful to Prof. Dr. Lothar Weber and Dr. Julia Bader for the helpful discussions. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




