Stereoinvertive SN1 Through Neighboring Group Participation
Abstract
Neighboring group participation, the assistance of non-conjugated electrons to a reaction center, is a fundamental phenomenon in chemistry. In the framework of nucleophilic substitution reactions, neighboring group participation is known to cause rate acceleration, first order kinetics (SN1), and retention of configuration. The latter phenomenon is a result of double inversion: the first one when the neighboring group displaces the leaving group, and the second when a nucleophile substitutes the neighboring group. This powerful control of stereoretention has been widely used in organic synthesis for more than a century. However, neighboring group participation may also lead to inversion of configuration, a phenomenon which is often overlooked. Herein, we review this unique mode of stereoinversion, dividing the relevant reactions into three classes with the aim to introduce a fresh perspective on the different modes of stereoinversion via neighboring group participation as well as the factors that control this stereochemical outcome.
1 Introduction
The term “neighboring group participation” (NGP) was coined by Winstein in 1942 to describe the assistance of electrons that are not conjugated to the reaction center.1 The phenomenon is characterized by rate acceleration and first order kinetics. In addition, control over stereochemistry is observed, usually resulting in retention of configuration, in contrast to the classical SN1 and SN2 reactions (Scheme 1a).2, 3 The retention of configuration is the result of double inversion, initially, when the neighboring group in 1 displaces the leaving group to form a positively charged intermediate 2, followed by a second inversion when the nucleophile displaces the neighboring group yielding 34 (Scheme 1b). In this review, the leaving group position is denoted as the α position (labeled in red), and the neighboring group position is denoted as the β position (labeled in blue).
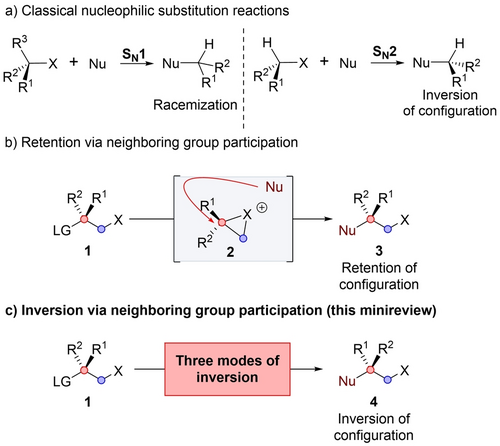
Nucleophilic substitution via neighboring group participation.
Over the years, retention of configuration has become well-known as an effect of NGP.5, 6 However, in contrast to this common belief, the stereochemical result of NGP is not limited to stereoretentive overall processes. There are several modes where inversion is the net result of NGP 4 (Scheme 1c). In this minireview, we classify these modes into three classes:
1.1 Inversion via Rearrangements
The neighboring group participates while inverting the α position, but the nucleophile attacks at the β position, resulting in skeletal rearrangement. In this section, we show illustrative examples that demonstrate the main factors controlling the regioselectivity (preference to attack at the β position over the α position).
1.2 Invertive Intermediate-Retentive Ring-Opening
Acyloxy groups or analogues (O, N, S) participate, displacing the leaving group with inversion at the α position, forming a cyclic acyloxonium ion. This intermediate is then hydrolyzed, yielding inverted product at the α position. In this section, we explain why the ring-opening is selective and how to distinguish this mechanism from the SN2 reaction that has the same stereochemical outcome.
1.3 Retentive Intermediate-Invertive Substitution
Acyloxy analogues participate with retention at the α position, followed by nucleophilic attack at this position, inverting the stereocenter. This class has unique features in contrast to the previous sections. These features and the ways to identify this mechanism are discussed in this section.
In this minireview, we show the basic mechanistic concepts of each class using representative examples, focusing on the stereo- and regioselectivity aspects.
2 Inversion via Rearrangements
Inversion via rearrangement is the inversion at both the α and β positions along with skeletal rearrangement. Nucleophilic substitution of the leaving group in 5 by the neighboring group inverts the α position, forming cationic intermediate 6. The latter is attacked by the nucleophile at the β position, inverting this position as well. Product 7 bears the neighboring group at the α position, implying rearrangement, and both the positions are inverted (Scheme 2). This mode is the most common pathway for stereoinversion by NGP.7 It is also applicable with a wide range of neighboring groups such as heteroatoms, arenes, cyclopropanes and norbornyl groups.8-14
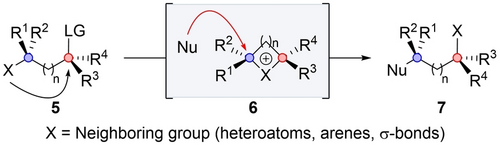
Inversion via rearrangements.
In these transformations, achieving regioselectivity towards the blue position during the nucleophilic attack on the cationic intermediate is crucial. The control of the regioselectivity could be thermodynamically controlled when the leaving group can act as the nucleophile, rendering the reaction reversible. This typically leads to the formation of the most stable product. Alternatively, the regioselectivity could be kinetically controlled when the irreversible nucleophilic reaction proceeds at the most reactive position. In this context, there are several parameters that govern regioselectivity and one should consider them to achieve the desired product. Among these parameters, the substrate characteristics, such as charge stabilization and steric hindrance, are pertinent factors affecting selectivity. Additionally, the nature of the neighboring group as well as the nature of the nucleophile also influence the outcome. The ultimate question is whether one can define general rules to predict the selectivity and when the rearrangement takes place.
To address these issues, we choose to showcase a few illustrative examples covering different neighboring group classes.
2.1 Heteroatom Participation
The first example of NGP was reported by W. P. Evans in 1891, with the participation of an oxygen heteroatom in the cyclization of chlorohydrin.15 This pioneering work did not consider the stereochemical aspects of NGP, but subsequent studies showed that the participation by heteroatoms can result in retention or inversion of configuration following the rearrangement explained above.8 In this section, we will examine representative cases involving common heteroatoms such as oxygen, nitrogen, and bromine. However, the principles discussed can potentially be applied to other heteroatoms that lead to inversion, like sulfur16 and chlorine.17
Nitrogen participation was shown by Southwick,18 when β-aminoketone 8 was converted to 10 through the formation of aziridinium intermediate 9, with a complete migration of the morpholine ring (Scheme 3a). In general, the control over the regioselectivity of the nucleophilic attack on aziridinium intermediates is sensitive to the exact reaction conditions including to the nature of the nucleophile.13 One way to rationalize the regioselectivity is by understanding the nature of the nucleophilic substitution, whether it resembles an SN2 or SN1 transformation, that have different factors controlling their selectivity.19 To determine the mode of the substitution, one must consider both the molecular pattern and the reaction conditions such as the nucleophile and solvent. In the particular case of intermediate 9, the mechanism of the nucleophilic attack has a SN1 character, with methanol acting as a weak nucleophile. Therefore, the regioselectivity is expected to be governed by the addition of the nucleophile at the carbon center that best stabilizes the positive charge. As anticipated, the attack occurs selectively at the benzylic position, rather than at the adjacent carbon center where the positive charge is destabilized by the presence of the carbonyl group.
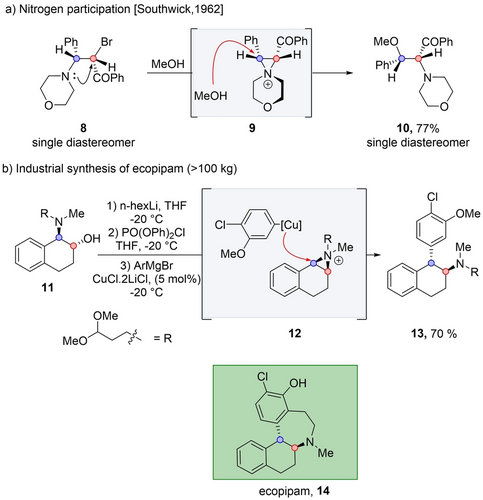
Rearrangement by nitrogen participation.
Nitogen participation is often used for drugs and natural product synthesis.20 A remarkable example is the pilot scale synthesis (>100 kg) of ecopipam 14, through aziridinium ion 12 (Scheme 3b).21 Amino alcohol 11 was converted into a phosphate leaving group followed by the formation of aziridinium intermediate 12. This intermediate was selectively attacked at the benzylic position by a Grignard reagent to form 13, which was converted into 14 over three steps. The nucleophilicity of the Grignard was increased by the addition of copper salt.
Stevens reported a cascade involving oxygen participation in mannose derivative 15.22 When 15 was treated with a solution of sodium acetate, a 1 : 7 mixture of diastereomers 17 a and 17 b was obtained (Scheme 4, condition A). These diastereomers products arise from the oxygen participation at C4, leading to the invertion of the center and formation of oxonium intermediate 16. This intermediate can undergo two distinct pathways: it may be attacked by acetate resulting in the formation of 17 a with inversion at both C4 and C5, or it can react with the mesylate to form intermediate 18, followed by a subsequent nucleophilic substitution reaction to provide 17 b, with inversion at C4 and retention (double inversion) at C5. Major product 17 b is presumably formed due to a rapid ion pair return. The regioselectivity of the ring-opening of 16 can be explained by considering the reaction at the carbon center that stabilizes the positive charge to a greater extent, in this case, C5 due to the inductive effect of the acetal oxygen which destabilizes the charge at C4. We hypothesize that for oxonium ion intermediates, the carbon center would accumulate more partial positive charge (relative to other heteroatoms intermediates), since oxygen does not accumulate positive charge well. Therefore, charge stabilization likely plays a significant role in determining regioselectivity.
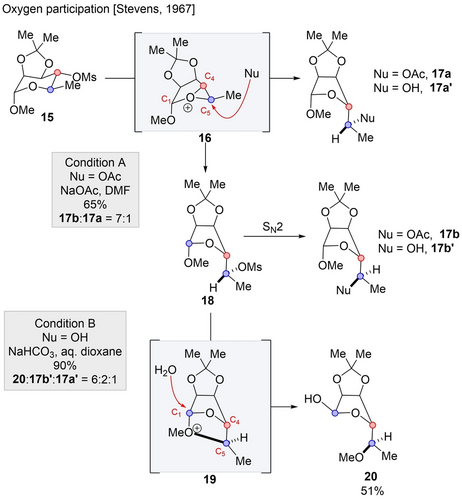
Rearrangement cascade by oxygen participation.
In a separate series of experiments, compound 15 was subsequently subjected to a dioxane aqueous solution in the presence of sodium bicarbonate (Scheme 4, condition B). In this case, the major product formed was 20 (20 : 17 b′:17 a′=6 : 2 : 1), obtained through a cascade reaction. Initially, intermediate 18 was formed via similar ion pair return, followed by the successive participation of the axial methoxy group to form five-membered oxonium intermediate 19. The hydroxide nucleophile selectively reacts at C1, resulting in the formation of 20 with inversion occurring at both C4 and C1. The attack occurs preferentially at C1, since the C1 substituent occupies an equatorial position. Here, these inversions occurred through two consecutive rearrangement events.
Bromine also effectively participates in such transformation through the formation of the well-established bromonium ion. This ion was first proposed to explain the selectivity of electrophilic addition of bromine to alkenes,23 and later was extended to NGP in nucleophilic substitution reactions.24 Braddock demonstrated that when compound 21 was treated with thionyl chloride, enantioenriched bromonium ion 22 was formed. Subsequent enantiospecific rearrangements led to the formation of 23 with high regioselectivity and without chiral erosion, while also inverting the configuration at both centers (Scheme 5).25 The regioselectivity results from a selective attack at the benzylic position. Interestingly, when the same reaction was performed on 24, it yielded an equimolar mixture of two products 26 and 27. This suggests that the reaction also proceeds through bromonium ion 25, but the attack at the intermediate is no longer selective, likely due to the insufficient electronic bias between the two reactive carbon centers. Moreover, the enantiomeric ratios of 26 and 27 are lower. The authors proposed that the racemization occurs due to the reaction of liberated chloride on the bromide, resulting in the formation of an alkene and BrCl. A non-selective addition of the latter into the alkene leads to the formation of two regioisomers 26 and 27 without selectivity.
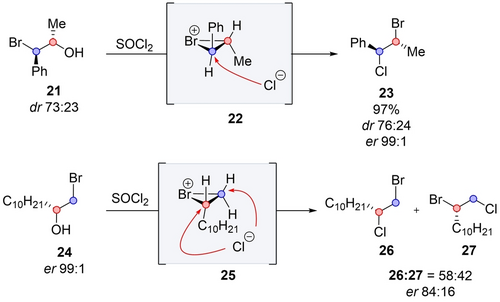
Rearrangement by bromine participation [Braddock, 2009].
2.2 Arene Participation
Phenyl group involvement in substitution reactions was first demonstrated by Cram in 1949, suggesting a mechanism proceeding via the participation of the aromatic ring through the formation of phenonium ion intermediate 29.26 By deuterium labeling experiments, it was indeed confirmed that upon treatment of 28 with formic acid, the formation of phenonium ion 29 occurred.27 This intermediate could equally react with a nucleophile at both the α and the β positions, resulting in an equimolar amount of 30 and 31 with complete scrambling of the deuterium (Scheme 6a). Although the substitution exhibited no selectivity in this case, stereoelectronic effects may favor the formation of a specific isomer, thereby selectively driving the transformation towards the rearrangement product.28 Weinges applied this concept to the regio- and stereoselective synthesis of rearranged chromane derivative 34 from 32 (Scheme 6b).29 Specifically, upon treatment of (+)-catechin tetramethyl ether 32 with PCl5, unsymmetrical phenonium intermediate 33 was initially formed. Subsequently, chloride selectively attacked this intermediate at the β position, leading to enantioenriched rearranged product 34 with inversion at both centers. Presumably, the selectivity arises from the attack occurring at the position possessing the highest charge stabilization, which in this case is the β position adjacent to oxygen. The reaction of epimeric starting material 35 did not provide 34, but instead elimination product 36, followed by an aerobic oxidation yielding benzopyrylium ion 37. In this case, rearrangement is not possible since the aryl group and the leaving group are not in an anti-relationship.
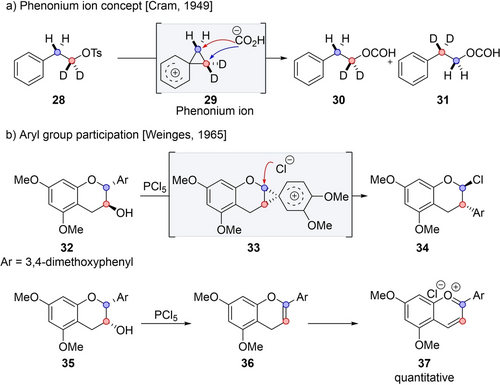
Rearrangement by arene participation.
2.3 σ Bond participation
The preceding sections focused on participation of lone pairs and π-bonds. Additionally, σ-bond participation has been also extensively documented and was initially proposed by Winstein to explain the solvolysis of norbornyl derivatives.30 Subsequently, Roberts expanded this concept to cyclopropyl carbinyl, cyclobutyl and homoallyl systems.31 The intermediates (38, 39) generated through σ-bond participation are known as non-classical carbocations (Figure 1).32, 33 These entities represent some of the most intriguing and intricate carbocations,34 and despite decades of investigation, their structure and reactivity remain not completely understood and predictable.
Non-classical carbocations.
These intermediates often enable rearrangements that significantly alter the carbon backbone. For instance, starting from cyclopropane, one can obtain either cyclobutane or homoallyl products. These rearrangements raise numerous questions, such as the exact structure of the intermediate, the relation between structure of intermediate and nature of the substituents, the cause of regioselectivity, the configurational stability of the intermediate, and whether the rearrangements are stereocontrolled. As previously mentioned, most of these questions remain unresolved. Due to the complex nature of these systems, we focus only on the rearrangement of the cyclopropyl carbinyl system. To begin addressing the aforementioned questions, we present recent examples that account for stereochemistry and provide mechanistic studies.
In recent years, our group has investigated the regio- and diastereoselective ring-opening of cyclopropyl carbinols 40 featuring stereodefined quaternary centers (Scheme 7a).35 Upon treating stereodefined cyclopropane 40 with a catalytic amount of Lewis acid, the addition of a nucleophile yields homoallyl product 42, with a complete inversion of configuration at the β-quaternary center (the α-center is converted into an alkene). This transformation can be conducted with a wide variety of nucleophiles, including alkyls,36 alcohols,38 carboxylates,35 halogens,35 nitrogen38- and sulfur39 based nucleophiles. Moreover, this transformation can be extended to the preparation of acyclic products with high enantioselectivity.36 Champagne computationally studied the aforementioned transformation using fluoride as the nucleophile, and rationalized the diastereoselectivity by proposing the formation of a stable cyclopropyl carbinyl cation (CC) intermediate 43, rather than the formation of a bicyclobutonium intermediate (BB) (Scheme 7b).40 The barrier for attack at the quaternary center (7.2 kcal/mol) was found to be the lowest among the four possible positions, addressing the question of regioselectivity. Furthermore, the epimerization of the stereocenters of 43, which could potentially proceed through an opening-closing sequence of the CC, was less favorable than the nucleophilic attack, thus accounting for the high diastereoselectivity.
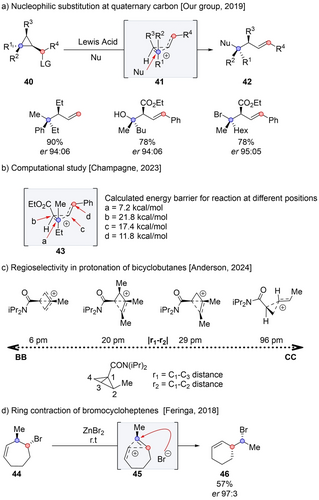
Rearrangement of bicyclobutonium, cyclopropyl carbinyl cation.
Subsequently, Champagne proposed a more comprehensive model at predicting whether a given cation is best described as a BB, CC or homoallyl cation.41 Results from this theoretical investigation indicate that the electronic nature of substituents, as well as their positions, affect regioselectivity. According to the structure of the intermediate, one should theoretically be able to rationalize the regioselectivity. However, this model was developed mainly for structures with only one substituent, and even when the structure of the intermediate is known, the distribution of products is not always clear. Anderson also introduced a model to account for the regioselectivity of the protonation of bicyclobutyl derivatives (Scheme 7c).42 According to this model, the structure of the intermediate can be viewed on a scale between BB and CC, determined by the difference between r1 and r2 bond lengths. If the structure leans closer to BB, the product will be a cyclobutane; conversely, if it leans closer to CC, the product will be a cyclopropane. If the structure falls in the middle of the scale, a mixture of products should result.
Feringa demonstrated the stereoinvertive homoallyl rearrangement of bromocycloheptenes 44 yielding bromoethyl cyclohexene 46 with up to 99 % enantiospecificity.43 Specifically, when enantioenriched 44 was activated by zinc bromide, chiral cationic intermediate 45 was initially formed and selectively attacked by bromide to provide 46. The barrier to obtain 46 from the reaction at 45 was lower than to revert to the starting material, indicating that the 46 is both the kinetic and thermodynamic product (cyclohexene being more stable than cycloheptene). Moreover, the barrier for this transformation is 7.8 kcal/mol, whereas the barrier to form a symmetrical BB intermediate with loss of stereochemical information is 10.8 kcal/mol. As a result, the overall process is enantiospecific, preserving the stereochemistry of the chiral non-classical carbocation. Interestingly, the same transformation could proceed on silica gel in the absence of a salt additive, albeit with a mechanism change into a concerted dyotropic rearrangement.
3 Invertive Intermediate-Retentive Ring-Opening
Acyloxy groups and their analogues (O,N,S) have unique modes of stereoinvertive participation besides rearrangements. These modes have been deemed “complex neighboring group participation”.44 In the next two sections, we classify the modes according to their mechanism.
The first mode is termed “Invertive Intermediate-Retentive Ring-Opening”. The reaction is initiated by the acyloxy group of 47, displacing the leaving group and inducing inversion of configuration at the α position (Scheme 8).45 Subsequently, in situ formed acyloxonium ion 48 undergoes ring-opening with retention of configuration at both centers, providing substitution product 49. This retentive ring-opening can be promoted by various nucleophiles, with hydrolysis being the most common. Altogether, this transformation results in inversion of configuration at the α center and retention at the β center. An important question, similar to the one raised in the previous section, is why the nucleophile selectively attacks at C3 rather than C1 or C2. Furthermore, the product resembles to a direct SN2 outcome, prompting the question of how to differentiate between the two pathways.
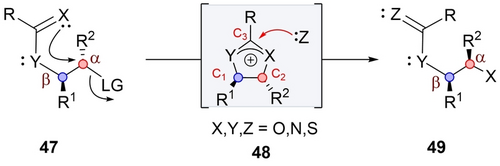
Invertive intermediate-retentive ring-opening.
This mode of participation was initially reported by Winstein in 1942, through the investigation of solvolysis and neighboring group participation using model substrate 50 (Scheme 9).45 Upon subjecting 50 to potassium acetate in dry acetic acid, anti-product 52 was obtained, resulting from the formation of acetoxonium intermediate 51, followed by attack of acetate ion at C1 or C2. However, altering the reaction conditions from dry to wet acetic acid, resulted in the formation of syn-product 53. This arises from the addition of water at C3 of 51, leading first to orthoester intermediate 54, which then collapses into 51. Furthermore, conducting the reaction in dry ethanol (or in dry acetic acid without potassium acetate) provided orthoester 55 (or 56), resulting from the addition of ethanol (or acetic acid) at C3. In subsequent investigations, Winstein showed that chloride ion behaved similarly to acetate, attacking the acetoxonium at C1 or C2.46 These experiments led to the conclusion that with nucleophiles such as chloride or acetate, the reaction at the intermediate is SN2-type in nature, occurring at C1 or C2 with an overall retention of configuration, while reaction with weak nucleophiles, such as water, alcohol and acetic acid, attack more akin to an SN1-type mechanism, occurring at C3.
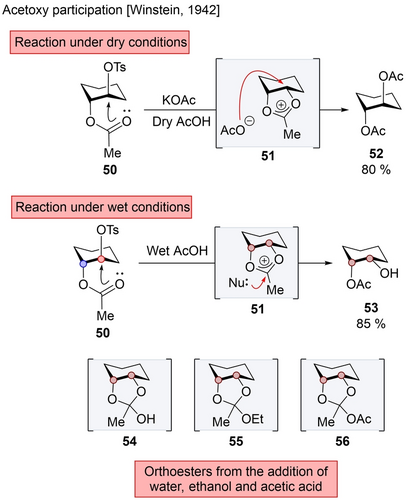
Acetoxy participation in cyclohexane derivatives.
After this pioneering work, this concept was utilized particularly in carbohydrate chemistry and extended to different acyloxy derivatives.10, 47 Goodman extensively studied this mode, especially considering the mechanistic questions. In one of his studies, mesylate 57, under sodium benzoate in refluxing DMF, was transformed into alcohol 59, inverting only the α position (Scheme 10).48 The authors proposed that the reaction proceeds through the formation of acetoxonium intermediate 58, which then reacts with water at C3 inducing the rearrangement of the resulting orthoester into product 56. This proposed NGP mechanism and a direct SN2 reaction (by benzoate) were not distinguishable since the product was isolated only after saponification. To answer this ambiguity, NMR and IR spectra of the crude mixture were measured which suggested the formation of a monohydroxybenzoate product, supporting the NGP pathway. Moreover, subjecting substrate 60, bearing a benzyl ether instead of the benzoate moiety, to the same reaction conditions, resulted in a sluggish reaction along with the formation of multiple products. This experiment showed that without the neighboring group, the reaction proceeded through direct SN2. In comparison, the NGP pathway was faster and cleaner and resulted in the hydroxy product as the major one.
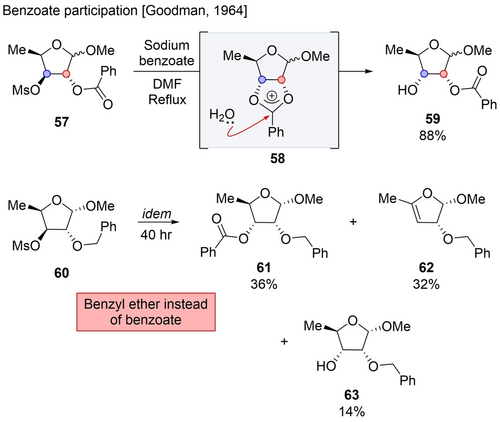
Rearrangement in carbohydrate series.
4 Retentive Intermediate-Invertive Substitution
The second mode is “Retentive Intermediate-Invertive Substitution”. In this mode, the acyloxy group in 64 participates with retention of configuration at the α position, through a tetrahedral intermediate which rearranges into acyloxonium ion 65 (Scheme 11). Subsequent rearrangement of the latter is ensued by a nucleophilic attack at the α position. This displacement restores the neighboring group at the β center while inverting only at the α center yielding 66. Several distinctive features set this mode apart from the preceding sections:
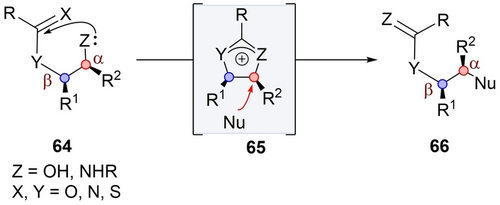
Retentive intermediate-invertive substitution.
-
The α center lacks a leaving group, instead featuring an alcohol (or amine), thereby preventing the possibility of direct SN2.
-
The participating group must exhibit a syn-relationship to the alcohol (or amine), also termed as “front side attack”.
-
The inversion occurs during the second step, facilitated by an intermolecular nucleophilic attack on the acyloxonium intermediate.
Winstein proposed this mechanistic pathway for the transformation of syn-diol 67 into 68 under rather harsh conditions (fuming hydrochloric acid and acetic acid, Scheme 12a).49 While a plausible alternative mechanism to interpret this transformation could involve a direct SN2 reaction via protonation of the leaving group (OH), Winstein dismissed this possibility since the reaction did not proceed in the absence of acetic acid, suggesting a possible crucial role of the acetate group (Scheme 12b). Moreover, when starting material 69, possessing an anti-relationship between the two diol functional groups, was subjected to the same experimental conditions, no chlorohydrin product was observed, although there is no major reasons for an SN2 reaction to proceed differently for syn- and anti-isomers (Scheme 12b). The mechanism is also unlikely to involve the formation of a monoacetate followed by acetate participation with protonated water as the leaving group (as in section 3). According to this mechanism the anti-isomer should be more reactive, while the syn-isomer was more reactive. Thus, Winstein proposed a mechanism that would start with the acetylation of one of the alcohols of 67 to provide 70. Then, an intramolecular reaction of the remaining alcohol would convert 70 into an orthoester intermediate through a “front side attack” (Scheme 12c). The orthoester would then rearrange into acetoxonium ion 51, which would subsequently undergo an intermolecular nucleophilic substitution by chloride to yield 68.
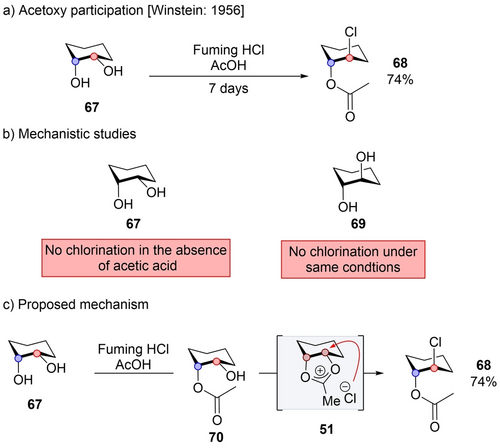
Acetoxy participation in cyclohexane derivatives.
Lichtenthaler showed the synthetic applicability of this mode in the synthesis of diamine derivative 73.50 When subjected to conditions involving acetyl bromide and acetic anhydride, amine 71 underwent conversion into bromide -73, with inversion of configuration at the α position (Scheme 13a). Lichtenthaler proposed that the mechanism operates via the front side attack, as suggested by Winstein.44 The hydroxy group in 72 initiates an attacks on the acetamide group, leading to the formation of a tetrahedral intermediate which subsequently rearranges into oxazolinium ion -71. This intermediate is further attacked by the nucleophilic bromide at the α position to provide bromide 73. To support this mechanistic hypothesis, a related derivative 74, treated under identical experimental conditions, provided bis-brominated product 75, indicating that bromination occurs at the two hydroxy groups adjacent to the acetamido groups (Scheme 13b). Moreover, when the acetamido and the hydroxy groups were in an anti-relationship, as in 76 no bromination is observed (Scheme 13c).
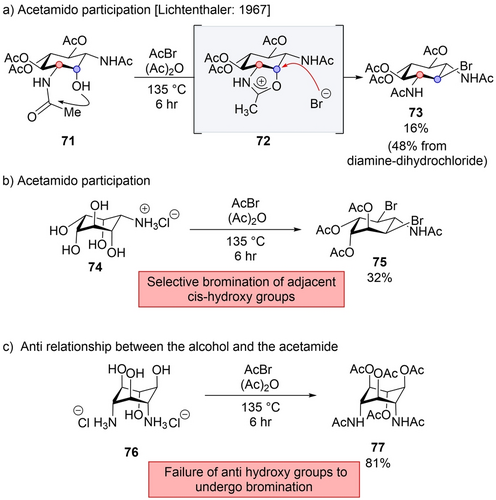
Synthetic application of retentive intermediate invertive substitution.
5 Conclusion
In this minireview, we have introduced a novel classification for the stereoinvertive nucleophilic substitution via neighboring group participation. Three modes have been categorized as follows: (1.1.) Inversion via Rearrangements; (1.2.) Invertive Intermediate-Retentive Ring-Opening and (1.3.) Retentive Intermediate-Invertive Substitution. In the first category, Inversion via Rearrangements (section 2), we have elucidated the fundamental principles governing regioselectivity towards rearrangement. These include discussions on the nature of the nucleophilic substitution on aziridinium intermediates and the role of charge stabilization in systems involving phenonium ions. It is important to note that within the context of σ-bond participation, numerous unresolved questions persist, and currently there is no overarching model that comprehensively predicts and explains the regioselectivity and stereoselectivity of these rearrangements. In Invertive Intermediate-Retentive Ring-Opening mode (section 3), we have discussed the conditions favoring this mode and methods to distinguish it from a direct SN2 pathway. Finally, we have illustrated the distinctive feature of Retentive Intermediate-Invertive Substitution (section 4), which contrast with the preceding modes. A few notable features include the absence of a conventional leaving group and the necessity for a “front side attack” to generate the reactive intermediate. We anticipate that the insights provided in this minireview will stimulate further investigations to address lingering questions and empower the synthetic community to expand upon these concepts to new horizons.
Acknowledgments
This project received funding from the Israel Science Foundation administrated by the Israel Academy of Sciences and Humanities (Grant No. 1625/22) and from the European Union's Horizon 2020 research and innovation program under Grant Agreement No 786976.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Biographical Information
Ilan Marek is a Distinguished Research Professor at Technion – Israel Institute of Technology. He holds the Sir Michael and Lady Sobell Academic Chair and is the director of the Resnick Sustainability Center for Catalysis. He is a member of the French Academy of Sciences, The Israel Academy of Sciences and Humanities and the Academia Europaea.
Biographical Information
Rahul Suresh graduated from the Indian Institute of Science, Bangalore in 2018. His Master work, under the guidance of Dr. Santanu Mukherjee focussed on the enantioselective halocyclization of olefins. Mr Suresh is currently pursuing his Ph.D. studies under the guidance of Prof. Ilan Marek. His studies focus on stereoselective synthesis using three membered rings.
Biographical Information
Noam Orbach completed his B.Sc. in chemistry at the Technion – Israel Institute of Technology through direct studies route to M.Sc. He joined the group of Prof. Ilan Marek to pursue his M.Sc. on the synthesis of polysubstituted bicyclobutanes and housanes. Then, he continued to a direct Ph.D. track in the group, focusing on the stereoselective synthesis using reactive intermediates derived from cyclopropenes.







