Unified Synthesis of 2-Isocyanoallopupukeanane and 9-Isocyanopupukeanane through a “Contra-biosynthetic” Rearrangement
Abstract
Herein, we describe our synthetic efforts toward the pupukeanane natural products, in which we have completed the first enantiospecific route to 2-isocyanoallopupukeanane in 10 steps (formal synthesis), enabled by a key Pd-mediated cyclization cascade. This subsequently facilitated an unprecedented bio-inspired “contra-biosynthetic” rearrangement, providing divergent access to 9-isocyanopupukeanane in 15 steps (formal synthesis). Computational studies provide insight into the nature of this rearrangement.
Nature has the ability to elaborate an early stage, “upstream”, biosynthetic intermediate into a wide variety of “downstream” secondary metabolites (natural products) through highly efficient and divergent biosynthetic pathways. Inspired by nature, chemists have continued to develop syntheses that provide unified access to structurally unique natural products from a common scaffold.1 Modern computational techniques have been shown to provide a deeper understanding of the potential energy surface for myriad biosynthetic pathways,2 which may now enable chemists to reverse the course of these pathways in order to access multiple natural products from a “downstream” biosynthetic intermediate—a “contra-biosynthetic” strategy.
Herein, we report unified formal syntheses of 2-isocyanoallopupukeanane (3, Scheme 1A) and 9-isocyanopupukeanane (1) by leveraging the allopupukeanane skeleton, a biosynthetically “downstream” intermediate, to access the “upstream” pupukeanane scaffold.
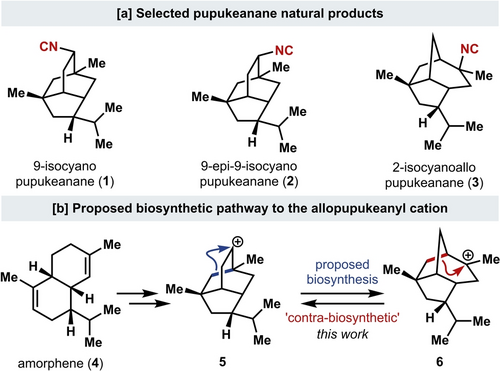
[a] Pupukeanane natural products. [b] Proposed biosynthesis of the pupukeanane and allopupukeanane skeleton (6).
The pupukeananes (selected members shown in Scheme 1A) are a family of structurally diverse marine-derived sesquiterpenes that possess caged tricyclic skeletons.3 The caged tricyclo[4.3.1.03,7]decane isotwistane core of a subset of these natural products has become known as the pupukeanane skeleton (1, 2, 5), while the tricyclo[5.2.1.04,8]decane scaffold of a related tricyclic congener is known as the allopupukeanane skeleton (3, 6). Many related natural products that possess diverse skeletons,4 as well as related functional groups,5 have also been isolated. Notably, there is renewed interest in isocyanoterpenes such as the pupukeananes due to their antimalarial activity, which is associated with a novel mechanism of action.6 Further understanding of this bioactivity could aid in combatting emerging issues of drug resistance.
Biosynthetically, the pupukeanane skeleton is proposed to arise from amorphene (4, Scheme 1B), which is converted to the pupukeanyl skeleton (5).4b, 4c, 7 The 9-pupukeanyl cation (5) is proposed to undergo a Wagner–Meerwein type shift to give the 2-allopupukeanyl cation (6). Trapping of carbocation intermediates (5 and 6) with cyanide8 would lead to the corresponding natural products 9-isocyanopupukeanane (1) and 2-isocyanoallopupukeanane (3), respectively. While many approaches to prepare natural products with the pupukeanane core have been reported,9 only two total syntheses of (±)-2-isocyanoallopupukeanane (3) have been achieved to date.10
We were draw to a synthesis of 3 given the low isolation yield of this biosynthetically “downstream” congener,11 as well as the lack of synthetic strategies to provide this natural product in enantioenriched form. We envisioned a short synthesis of 3 using strategic C−C bond disconnections that provide the opportunity to access the related pupukeanane natural products (Scheme 2). Specifically, we anticipated that carbocation 6 would lead to 3 through direct cyanide capture, whereas a late-stage “contra-biosynthetic” rearrangement of 6 could yield 5 and ultimately 9-isocyanopupukeanane (1). Carbocation 6 could arise from corresponding alkene 7, which in turn would be prepared from 8 through an aldol condensation, in the forward sense, to forge the tricyclic allopupukeanene core. Key to our synthetic strategy was rapid access to the bicyclo[3.2.1]octane framework of 8. Given our longstanding interest in the rearrangement of readily available, chiral, enantioenriched terpenes to access diverse scaffolds,12 we envisioned an approach that leveraged the C−C cleavage of a strained cyclobutanol.13 Specifically, we envisioned 9 undergoing sequential ring-opening and Mizoroki-Heck-type cyclization. Cyclobutanol 9 was envisioned to arise in 3 steps from (R)-carvone (10), an inexpensive starting material that is readily available in both enantiomeric forms and can be used to access a variety of natural products with unique skeletons.14
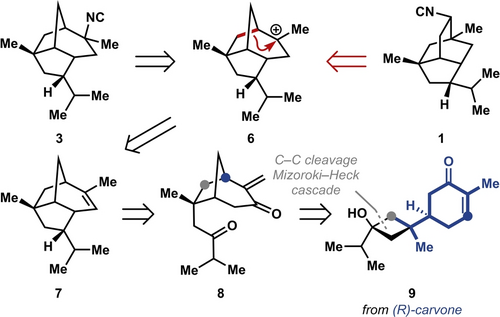
Our proposed retrosynthesis of 2-isocyanoallopupukeanane (3) and 9-isocyanopupukeanane (1).
Our synthetic route commenced with the known [2+2] cycloaddition of in situ generated dichloroketene to the isopropenyl group of (R)-carvone (10)15 and subsequent reductive dechlorination to provide cyclobutanone 11 (Scheme 3). At this stage, we attempted the addition of isopropyl magnesium bromide. Addition of Grignard reagents to 11 led to non-selective mono- and double-addition products. However, treating enone 11 with triphenylphosphine (PPh3) and triethylsilyl trifluoromethanesulfonate (TESOTf) effected in situ protection of the enone moiety,16 which enabled the addition of isopropyl lithium (i-PrLi) to the cyclobutanone carbonyl group. In the same pot, the enone was unmasked following addition of the nucleophile, to provide cyclobutanols 9 and 9′ as a mixture of anti- and syn-addition adducts. This sequence could be scaled to provide gram quantities of 9 and 9′ in a single pass.
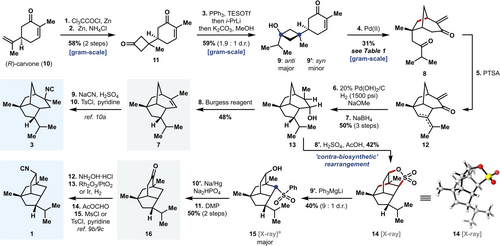
Formal synthesis of 2-isocyanoallopupukeanane (3) and 9-isocyanopupukeanane (1). [a] See Supporting Information for further details.
With cyclobutanol 9 in hand, we turned our attention to the proposed Pd-mediated C−C bond cleavage/Mizoroki–Heck cascade reaction that would yield [3.2.1]bicycle 8 (Table 1).13b We hypothesized that a palladium(II)-complex would first undergo X-type ligand exchange with the tertiary hydroxy group of cyclobutanol 9 (see I1) followed by a diastereo-determining β-carbon elimination to form alkyl palladium intermediate I2. Given the absence of a β-hydrogen in I2, this alkyl palladium intermediate was anticipated to undergo a Mizoroki–Heck-type migratory insertion across the alkene of the enone to form the corresponding palladium enolate (I3). Finally, a β-hydride elimination would yield the exocyclic enone diastereomers 8 and 8′.
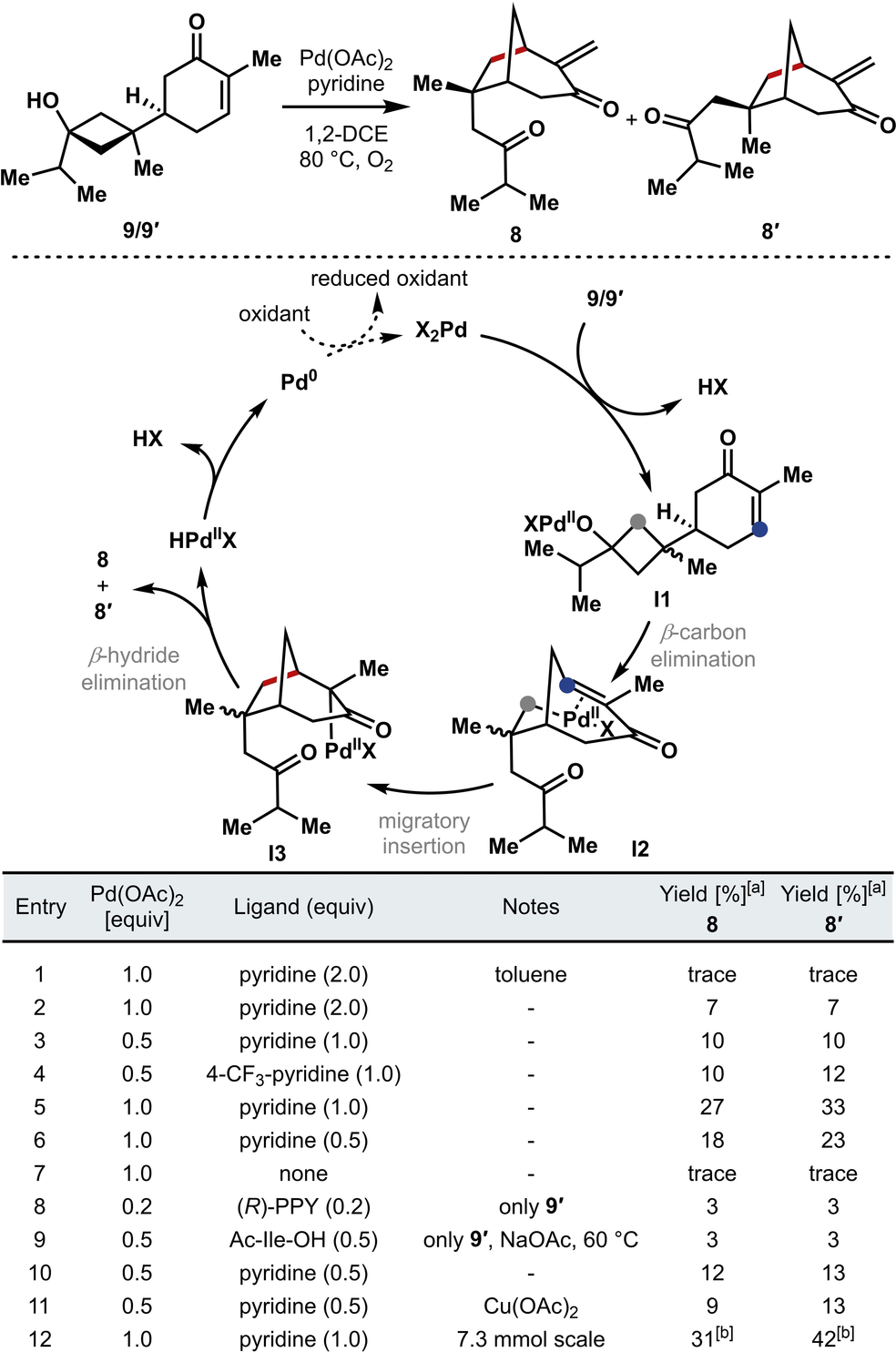
- [a] Determined by 1H NMR analysis of a 0.0085 mmol (2 mg) scale reaction using 1,3,5-trimethoxylbenzene as an internal standard. [b] Isolated yields.
Application of the conditions disclosed by Uemura in the seminal report of cyclobutanol opening through a Pd(II)/Pd(0) cycle,13b gave rise to small amounts of the desired bicycle (Table 1, entry 1). Using 1,2-dichloroethane as solvent led to an increase in yield (Table 1, entry 2). Of the ligands that were screened for this transformation, only nitrogen-based ligands led to the desired product. We also observed that electron-deficient pyridines were competent ligands that led to a comparable outcome (Table 1, entry 3–4). However, we chose to proceed with pyridine as the ligand of choice due to the comparatively low cost. The ratio of palladium to pyridine was crucial to the success of this reaction (Table 1, entries 5–7). We also examined the diastereoselective opening of cyclobutanols 9 and 9′. Historically, the stereoselective β-carbon elimination of cyclobutanols such as 9 has proven challenging,13b although limited examples have been reported.17 Using chiral, enantioenriched ligands (e.g., (R)-PPY; see the Supporting Information for further details) under our reaction conditions with cyclobutanol 9′, we observed a 1 : 1 ratio of 8 and 8′, pointing to a lack of diastereo-differentiation of the methylene groups under these conditions (Table 1, entry 8).18 Employing ligands developed by the Yu group for similar Pd-mediated oxidative processes19 (Table 1, entry 9) led only to low yields of a mixture of 8 and 8′ as well.
To date, we have not been able to obtain high yields of the desired Pd(II)-mediated cascade product (i.e., 8) using sub-stoichiometric amounts of Pd(II) salts (Table 1, entry 10). In many previous reports of transformations that proceed through a Pd(II)/Pd(0) catalytic cycle, an excess of the pyridine ligand was vital to maintaining Pd(0) in solution without precipitation as Pd-black.13a, 20 However, in our case, we observed that a 1 : 1 ratio of Pd(II) salt:ligand gave the best results and product formation was not observed in cases where a large excess of pyridine was used. While we would still expect the active Pd species to be bis-ligated under these conditions,21 presumably, an excess of pyridine hinders the ligand dissociation required to promote the β-carbon elimination/migratory insertion sequence. In our studies, even the introduction of external oxidants (Table 1, entry 11), did not improve conversion. Despite recent progress toward improved speciation in Pd(II)/(0) catalytic cycles,22 it is well-known that reoxidation of Pd(0) intermediates is often slow and can fail due to the precipitation of Pd black;22b, 23 efforts to improve the outcome of such reactions in the context of the transformation of 9/9′→8 are ongoing in our laboratory. However, the protocol that is currently employed is scalable and provides bicycle 8 in sufficient quantities for our syntheses of the pupukeanane molecules (Table 1, entry 12).
From key bicycle 8, an aldol condensation mediated by p-toluenesulfonic acid (PTSA) forged the tricyclic core of allopupukeanane (12, Scheme 3) as an inconsequential mixture of alkene isomers. Global hydrogenation at this stage, followed by sodium borohydride (NaBH4) reduction of the resulting ketone group yielded alcohol 13. However, this reaction was quite sluggish and mono-hydrogenation products were often isolated after extended reaction times under high pressure of hydrogen. We hypothesized that the reduction of the mixture of tri- or tetra-substituted alkenes was hindered by a steric clash on the concave face of the bridged tricycle between an α-disposed methyl group, which results from reduction of the enone, and the developing α-disposed isopropyl group. The addition of NaOMe to the hydrogenation reaction mixture overcame this challenge through epimerization to place the methyl group on the β-face. Following reduction of the resulting ketone with NaBH4, alcohol 13 (an intermediate in Ho's synthesis of 7) was isolated as the major product in 50 % yield over 3 steps along with trace quantities of other compounds presumed to be epimers of 13.
From secondary alcohol 13, a dehydration (not previously reported) mediated by the Burgess reagent gave volatile allopupukeanene 7. Ho and co-workers previously reported the conversion of 7 to 2-isocyanoallopupukeanane (3) through addition of HCN across the alkene in a Ritter reaction and subsequent dehydration of the resulting formamide to yield 3.10a In summary, allopupukeanene 7 was prepared in 8 steps from (R)-carvone and constitutes a 10-step formal synthesis of 2-isocyanoallopupukeanane (3)—shortened from both Ho and Tanino's syntheses of the racemate, each consisting of 17 steps.
At this stage, we turned our attention to effecting the rearrangement of the allopupukeanane framework (i.e., 13) to the pupukeanane core. We anticipated that this transformation would be difficult to accomplish, since trapping of the pupukeanane skeleton would require the formal capture of a secondary carbocation (5) that is unlikely to be a minimum on the potential energy surface.2a, 7, 24 However, we speculated that the desired transformation might be possible through a concerted Wagner–Meerwein shift and nucleophilic capture of a kinetically generated allopupukeanyl cation (6).
Indeed, formation of the pupukeanane core from an allopupukeanyl precursor proved particularly challenging. Our initial attempts led only to decomposition or capture of 6 by the exogenous nucleophile. Therefore, we sought to gather further mechanistic insight through computational modeling. Calculations were conducted on the capture of 6 with a nucleophilic cyanide source, trimethylsilyl cyanide (Scheme 4, left), using the Gaussian 16 software25 and the ωB97X−D(SMD)/def2-SVP level of theory (see the Supporting Information for further details).26 The corresponding transition structure for TMSCN addition was located (A-TS1) and, as expected, the direct capture of 6 to give 2-isocyanoallopupukeanane (3) was calculated to be both kinetically and thermodynamically feasible. Additionally, the 9-pupukeanyl cation (5; Scheme 4, inset) was not observed to be a minimum on the potential energy surface. We were unable to locate an alternative transition structure for concerted attack of TMSCN and alkyl shift (B-TS1), despite the fact that 9-epi-9-isocyanopupukeanane (2) was predicted to be thermodynamically more stable than the 2-isocyanoallopupukeanane congener (3).
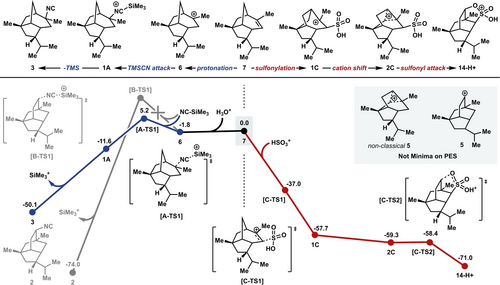
Computational studies on the rearrangement of the allopupukeanane core (7).27
When allopupukeanene 7 was subjected to sulfuric acid (H2SO4) and glacial acetic acid (AcOH) at elevated temperatures, we observed the formation of small amounts of pupukeanyl sultone 14 (Scheme 3). The sulfonation of alkenes is known and constitutes an important step in the formation of camphor sulfonic acid.28 Furthermore, simple sultones have been formed through the addition of electrophilic SO3 across an alkene followed by Wagner–Meerwein type rearrangements.29 Ultimately, we were able to improve upon our initial observation by accessing sultone 14 directly from alcohol 13 upon treatment with a mixture of H2SO4 and AcOH. This alternative sequence avoided the isolation of volatile allopupukeanene 7. Presumably 13→14 proceeds through an acid-mediated elimination of the hydroxy group to give allopupukeanene 7 followed by an electrophilic sulfonation and subsequent Wagner–Meerwein rearrangement, selectively trapping the less thermodynamically stable secondary carbocation by intramolecular nucleophilic attack of the sulfonic acid group. The structure of 14 was unambiguously confirmed by single crystal X-ray diffraction.
On the basis of these empirical findings, we modeled the reaction coordinate for the sulfonation of 7 with an activated sulfur electrophile to form intermediate 1 C (Scheme 4, right). From this intermediate, the rearrangement is nearly barrierless, proceeding through a nonclassical carbocation (2 C)30 on a flat region of the potential energy surface to reach protonated pupukeanyl sultone 14-H+ as an energy minimum. Thus, it would appear that while a direct capture of the 9-pupukeanyl cation (5) is not feasible, by coupling the generation of the carbocation with a sulfonation process, we are able to trap the pupukeanane skeleton in the form of pupukeanyl sultone 14.31 This transformation is effectively the reverse of the proposed biosynthetic pathway, accessing the pupukeanane core from a “downstream” biosynthetic intermediate.
We then turned our attention toward the synthesis of 9-isocyanopupukeanane (1, Scheme 3), however further manipulation of sultone 14 proved extremely challenging. Applying known methods to directly reduce or open aliphatic sultones32 failed in our hands. Inspired by Fleming and co-workers’ sulfone-metal exchange strategy,33 we investigated the addition of lithium magnesiates to sultone 14. While sulfur-metal exchange would not be expected for a sultone, owing to the presence of an alkoxide leaving group, treating sultone 14 with lithium triphenyl magnesiate (Ph3MgLi)34 afforded ring-opened phenyl sulfone 15, now bearing a suitable electron-sink to aid C−S bond reduction. We speculate that the major diastereomer of phenyl sulfone 15 (confirmed through single crystal X-ray diffraction)35 arises from kinetic protonation of the alkoxide-chelated α-sulfone magnesiate upon work-up. Notably, use of phenyl magnesium chloride (PhMgCl) or diphenyl magnesium (Ph2Mg) instead of Ph3MgLi led to recovery of starting material, while treatment with phenyl lithium (PhLi) resulted in decomposition (see the Supporting Information for further details), highlighting the narrow threshold for productive reactivity of sultone 14. Reduction of phenyl sulfone 15 with sodium/mercury amalgam (Na/Hg) cleaved the desired C−S bond, and subsequent oxidation of the resulting secondary alcohol with Dess–Martin periodinane (DMP) afforded 9-pupukeanone (16) in 11 total steps from (R)-carvone, constituting a 15-step, enantiospecific formal synthesis of 9-isocyanopupukeanane—the shortest synthesis to date.9
In conclusion, we have developed a unified strategy for the concise enantiospecific formal synthesis of 2-isocyanoallopupukeanane and 9-isocyanopupukeanane (10 and 15 steps from (R)-carvone, respectively). Key features of the synthesis include a Pd-mediated cascade to form a [3.2.1]bicycle followed by an aldol condensation/cyclization to build the tricyclic carbon skeleton of allopupukeanane in 2 steps from a readily accessed chiral-pool derivative. The short synthetic sequence has enabled us to explore the proposed biosynthetic pathway to these architecturally fascinating molecules and the corresponding potential energy surface. These efforts culminated in the development of a “contra-biosynthetic” rearrangement from a “downstream” biosynthetic intermediate to access the pupukeanane skeleton in the form of a sultone. The utility of this “contra-biosynthetic” approach was then demonstrated through the formal synthesis of 9-isocyanopupukeanane. This unified synthetic approach should prove useful in accessing many pupukeanane and allopupukeanane natural products, as well as their analogs, for testing as potential antimalarial compounds. Ongoing studies are focused on optimizing the Pd-mediated cyclization cascade and accessing a variety of additional natural products from late-stage intermediates.
Acknowledgments
R.S. is grateful to the National Institutes of General Medical Sciences (R35GM130345) and the NSF Center for Computer Aided Synthesis (CCAS; CHE-1925607) for funding. M.A.H. is grateful for funding from the NSF graduate research fellowship program (DGE-1752814). K.N. is grateful for funding from the JSPS predoctoral fellowship (22KJ0222) and the Tohoku Kaihatsu Memorial Foundation. D.J.T. and Z. F. gratefully acknowledge support from NSF (CHE-1856416 and computational resources from the XSEDE program). We kindly acknowledge the Swiss National Science Foundation for an Early Postdoc. Mobility Fellowship to support I.K. (P2BSP2_168732) and the Amgen Scholars Program for a summer fellowship to L.A.M.. Additionally, we are grateful to Dr. Cedric L. Hugelshofer (Merck) for helpful conversations regarding structure elucidation, and to Prof. Hosea Nelson, Christopher Jones, and Jessica Burch (UCLA) for insightful discussions regarding small molecule crystallography. We thank Dr. Nicholas Settineri for single crystal X-ray diffraction studies and Dr. Hasan Celik and College of Chemistry's (CoC) NMR facility for spectroscopic assistance. Instruments in CoCNMR are supported in part by NIH S10OD024998.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




