A Fluorogenic DNAzyme for A Thermally Stable Protein Biomarker from Fusobacterium nucleatum, a Human Bacterial Pathogen
Abstract
Fusobacterium nucleatum has been correlated to many poor human conditions including oral infections, adverse pregnancies and cancer, and thus molecular tools capable of detecting this human pathogen can be used to develop diagnostic tests for them. Using a new selection method targeting thermally stable proteins without a counter-selection step, we derived an fluorogenic RNA-cleaving DNAzyme, named RFD-FN1, that can be activated by a thermally stable protein target that is unique to F. nucleatum subspecies. High thermal stability of protein targets is a very desirable attribute for DNAzyme-based biosensing directly with biological samples because nucleases found inherently in these samples can be heat-inactivated. We further demonstrate that RFD-FN1 can function as a fluorescent sensor in both human saliva and human stool samples. The discovery of RFD-FN1 paired with a highly thermal stable protein target presents opportunities for developing simpler diagnostic tests for this important pathogen.
Introduction
The gram-negative anaerobe Fusobacterium nucleatum (FN) is often found as part of the human oral flora, as subgingival plaque.1 Although commensal, FN’s virulence factors, like the adhesins FadA and Fap2,2 can promote bacterial pathogenicity, and allow it to proliferate within its host. Over time, molecular events initiated by FN can contribute to conditions like periodontitis (gum disease), adverse pregnancies, gastrointestinal disorders, cancer, Alzheimer's disease, Lemierre's syndrome, cardiovascular disease, rheumatoid arthritis, and respiratory tract infections.3 Therefore, FN is an important human pathogen in which detection can help mitigate disease through proper clinical interventions by clinicians.
The current gold standard for FN detection is cell culture, with polymerase chain reaction (PCR) as a promising alternative.1, 4 Despite the fact that both of these methods are able to accurately detect FN, they fail to provide a rapid and simple detection platform. Cell culture requires an incubation step (96 hours), followed by multiple biochemical tests, all of which are time-consuming and laborious. PCR-based methods offer shorter detection times, but they require the use of expensive, laboratory-based equipment and an additional DNA preparation step. There exists a great need for the development of simpler detection methods for FN. Synthetic DNA-based enzymes (deoxyribozymes or DNAzymes), man-made single-stranded DNA molecules with catalytic activities, are well suited to address this need by acting as molecular recognition and reporting elements for assay development.
A variety of DNAzymes have been reported to catalyze many different chemical reactions.5 In recent years, analyte-responsive (or target-activated) DNAzymes have garnered considerable attention as biosensing molecules and diagnostic agents due to their ability to recognize a target of interest with high sensitivity and specificity.6, 7 A specific class of DNAzymes, known as RNA-cleaving fluorogenic DNAzymes (RFDs), in which RNA cleavage activity is programmed with fluorescent signal generation, have been selected for a handful of bacteria,8 including Escherichia coli, Clostridium difficile, Helicobacter pylori, Klebsiella pneumoniae, Legionella pneumophila, and Staphylococcus aureus. These signaling DNAzymes have been derived from random DNA libraries using a process known as in vitro selection.9 It has been shown that RFDs can be used as the key component of biosensing systems for bacterial detection.7, 10 For example, RFD-EC1, an RFD activated by E. coli, has been incorporated into multiple biosensing platforms, some of which are capable of detecting as few as 10 colony forming units (CFUs) of E. coli in complex matrices.10 Given these advancements, opportunity exists for deriving RFDs for other important bacterial pathogens, such FN in the current work.
Herein we report on the selection of RFD-FN1, an RFD that exhibits highly selective RNA-cleaving activity towards FN. This DNAzyme was derived using a significantly altered (simplified) in vitro selection strategy in two aspects. First, in our previous RFD selection studies, we always used crude extracellular mixture (CEM) produced from cell culture as the complex target to search for RFDs that can be activated by a specific biomarker in the mixture.7 Given that nucleases (DNases and RNases) that can degrade DNAzymes are abundant in biological systems, we were curious if we could introduce a heat-denaturing step where the CEM would be treated at a high temperature (such as 90 °C) for a period of time to achieve two goals: deactivating nucleases and targeting a thermally stable protein biomarker. These two points were clearly demonstrated with the CEM prepared with FN (CEM-FN), which degraded a fluorogenic substrate S1 in an hour at 37 °C (Figure S1; the sequence of the S1 substrate was provided in Figure S2); however, the S1 substrate experienced very little degradation under the same reaction conditions with CEM-FN that was heated at 90 °C for 15 minutes (Figure S1). Second, our previous efforts always used a counter-selection step where the DNA pool was incubated with one or more control bacteria prior to the positive selection with an intended bacterium, with the premise that double negative/positive selection was necessary to eliminate DNAzymes with cross-activities towards unintended bacteria. Although this strategy has been associated with the identification of several highly specific RFDs for bacterial recognition, the need for a vigorous counter-selection step has not been proven. In the current study, we did not include a counter-selection step; interestingly, without this step, we were still able to derive a DNAzyme that is highly specific for FN. More remarkably, the incorporation of a vigorous heat denaturation step led to the discovery a DNAzyme that targets a thermally stable protein for recognition.
Results and Discussion
FN-specific RFDs were isolated via the selection strategy illustrated in Figure S2 (all experimental details are provided in Section 1 of Supporting Information). The starting DNA pool contained ~1014 random DNA sequences, which was designed to contain a random-sequence domain of 50 nucleotides (nt), a 20-nt primer-binding site on each side of the random domain, and a 30-nt fluorogenic substrate (Figure 1A). The substrate sequence (named S1) contains a single ribonucleotide (adenosine) at position 14, where the cleavage occurs. The ribonucleotide is flanked by a pair of thymine deoxyribonucleotides modified with fluorescein (F; 15th position) and the DABCYL quencher (Q; 13th positive). Upon RNA cleavage, two products would be produced: a fluorescein-containing DNA fragment (FP) and a quencher-bearing fragment (QP). An increase in fluorescence was expected due to dequenching (Figure 1B). Prior to selection, FN subspecies (ssp.) nucleatum was cultured anaerobically to an OD600nm reading of approximately 0.7 and the cells were removed by centrifugation. The resulting crude extracellular mixture (CEM), herein referred to as CEM-FN, was used as a mixture of possible targets for selection. CEM-FN was treated at 90 °C for 15 minutes. The DNA library was first ligated to the fluorogenic substrate (FQ30) and incubated in 1× selection buffer (1× SB; 50 mM HEPES, 150 mM NaCl, 15 mM MgCl2, and 0.01 % Tween 20, pH 7.5) for 2 hours to remove self-cleaving RFDs that utilize the components of SB for cleavage, such as metal ions. The active molecules in this step were discarded, and the inactive DNA sequences were purified by 10 % denaturing (7 M urea) polyacrylamide gel electrophoresis (dPAGE) and incubated with heat-treated CEM-FN in 1× SB for 3 hours. This served as the target-activation step where RFDs that were responsive to a biomarker in CEM-FN were isolated and purified using 10 % dPAGE. The cleaved sequences were then amplified by PCR and used for the subsequent round of selection.
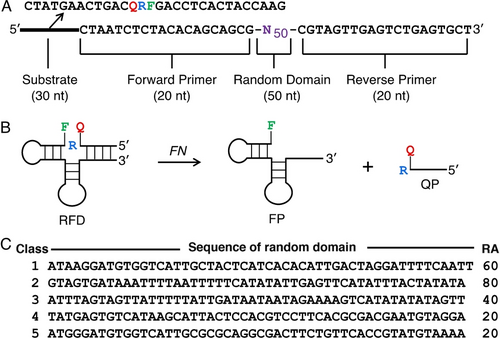
In vitro selection of RNA-cleaving fluorogenic DNAzymes (RFDs) for FN recognition and signal generation. (A) The sequence of the DNA library used in this study. R: adenosine ribonucleotide; F: fluorescein-labelled dT; Q: DABCYL-labelled dT; N: mixture of 25 % A, 25 % G, 25 % C, 25 %T. (B) Schematic of the cleavage reaction of cis-acting RFDs. (C) The random-sequence domain of the top 5 classes of selected RFDs. The class 2 sequence is named RFD-FN1. RA: relative activity, estimated as CCEM-FN/CSB; CCEM-FN=% cleavage in CEM-FN; CSB=% cleavage in 1× SB only.
Nine rounds of selection and amplification were carried out. In the 9th round, a strong cleavage activity was observed in the presence of CEM-FN relative to the negative control, which contains only 1× SB (Figure S3). The Illumina MiSeq platform was used to identify the enriched active DNA sequences. Sequencing reads were clustered into classes based on sequence similarity and ranked by the number of sequences in each class to identify the dominating sequences in the pool (Figure 1C).11 The relative activity (RA) values of the top five classes were analyzed, the class 2 sequence, herein referred to as RFD-FN1, displayed the highest cleavage activity and was chosen for further investigation.
The original RFD-FN1 was isolated to cleave a covalently attached substrate sequence (i.e., cis-acting). However, a trans-acting RFD-FN1 DNAzyme would provide additional advantage of ease of preparation because the DNAzyme sequence is no longer linked to the substrate sequence, which removes the ligation step. Upon careful inspection of the secondary structure of cis-acting RFD-FN1 for potential base-pairing interactions, we identified three duplex elements, which are labeled as P1, P2 and P3 in Figure S4. To make a trans construct, the substrate domain (nucleotides 1 to 30 of cis-acting RFD-FN1) was detached from the DNAzyme sequence (nucleotides 31 to 120). The catalyst strand was then broken down into three sequence segments according to the predicted secondary structure shown in Figure S4: D1, D2 and D3 (Figure 2A). These segments were used to set up 4 trans-acting constructs named DT1 to DT4. DT1 represents the full-length construct (made of all three segments) while DT2 is made of D1 and D2, DT3 is made of D2 and D3, and DT4 is made of D2 only. The shortest construct, DT4, where the DNAzyme strand is made of the first 38 random-sequence nucleotides of RFD-FN1, displayed an improved activity (16 % cleavage in DT4 vs. 5 % cleavage in full-length construct DT1 in 1× SB at E/S=10/1, 23 °C, 4 hours). These results indicate that D1 (the loop of the P2 hairpin) and D3 (the unstructured sequence element at the end of RFD-FN1) are disposable for the function of the DNAzyme. This is not a surprising finding because most of the nucleotides in D1 and D2 fall within the two constant sequence elements of the initial library that serve as the primer binding sites for PCR.
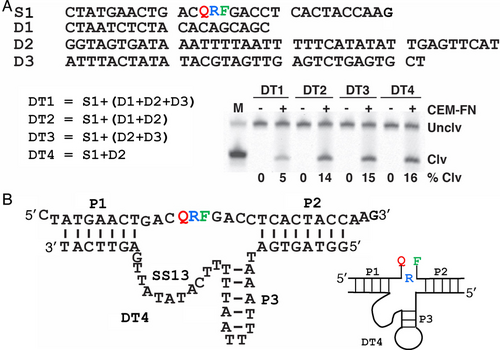
Sequence and secondary structure properties of RFD-FN1. (A) Cleavage activities of the trans-acting DNAzyme constructs DT1 to DT4; S1: the fluorogenic substrate FQ30. Unclv: uncleaved S1; Clv: the cleavage fragment of S1 carrying F; M: marker lane. (B) The proposed secondary structure of DT4. P: pairing region; SS13: single-stranded motif linking P1 and P3. The simplified drawing of the secondary structure of DT4 is shown on the right. DNAzyme assays were conducted at 23 °C in 1× SB at E/S=100/1 for 4 hours. The uncropped image of the gel in this Figure is provided in Figure S12A in the Supporting Information.
The proposed secondary structure of DT4 (Figure 2B) is reminiscent of many reported RNA-cleaving DNAzymes, represented by the well-known RNA-cleaving DNAzymes 8–17 and 10–23.12 P1 and P2 serve as substrate-binding arms while P3 acts as part of catalytic core. Interestingly, the suspected catalytic core of DT4, comprised of P3 and SS13, is rich in A and T bases (91.7 %); out of 24 nucleotides, 14 are thymine (58.3 %) and 8 are adenine (33.3 %). To our knowledge, DNAzyme sequences of this nature have never been reported before.
Divalent metal ions have been known to play important roles in the activity of RNA-cleaving DNAzymes. The effects of different divalent metal ions on DT4 were tested (Figure S5A). It was found that DT4 displayed a strong activity only in Mg2+, which was the metal ion included in 1× SB. In contrast, Mn2+ and Co2+ induced weak cleavage while Ca2+, Ni2+, Cu2+, Zn2+, Cd2+ and Ba2+ did not induce any cleavage. The effect of different Mg2+ concentration on DT4 activity was also tested and it was found that the highest activity was achieved with a final Mg2+ concentration range of 7.5–15 mM (Figure S5B).
We also examined the cleavage activity of DT4 under different pH and reaction temperatures. We found that highest activity was achieved at pH 7.5 (Figure S6) and 23 °C (Figure S7), both of which were the original reaction condition used during selection.
DT4 was isolated to cleave in the presence of CEM-FN. It is conceivable that the target that activates this DNAzyme also exists inside cells, as previously shown by RFD-EC1, the DNAzyme selected to be activated by E. coli.8a This was tested by performing the following experiment: FN cells were first cultured and then pelleted by centrifugation. The supernatant was taken as CEM-FN. The cell pellet was re-suspended in 1× SB and then heated at 90 °C for 15 minutes to disrupt the cell membrane and release intracellular content. The sample was centrifuged to pellet cell debris and the resulting supernatant was labeled as CIM-FN (CIM stands for crude intracellular mixture). Both CEM-FN and CIM-FN were then used to induce the cleavage activity of DT4 (Figure 3A). This experiment revealed that the target for DT4 exists both inside and outside of cells.
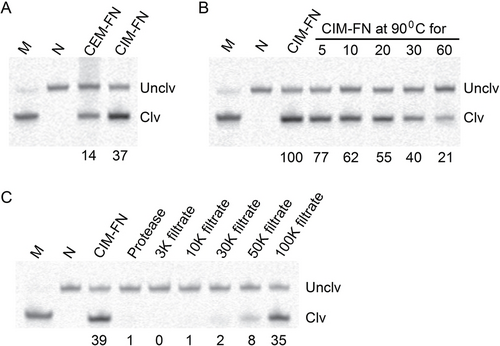
Target characteristics of the activator for DT4. (A) Activity of DT4 with CEM-FN and CIM-FN. (B) Activity of DT4 with heat treated CIM-FN at various times. RA: relative activity, calculated as Ct/C0; Ct=% cleavage of DT4 in CIM-FN treated at 90 °C for a given time; C0=% cleavage of DT4 in CIM-FN without heat treatment. (C) DT4 with protease-treated CIM-FN and filtered CIM-FN. CIM-FN was passed through centrifugal devices with a specific molecular weight cut-off individually and tested for cleavage activity. All DNAzyme assays were conducted at 23 °C in 1× SB at E/S=100/1 for 4 hours. M: marker, N: negative control (no reaction control). The uncropped images of the three gels in this figure are provided in Figure S12 (panel B, C and D, respectively) in the Supporting Information.
Remarkably, DT4 exhibited a high-level activity in CIM-FN, despite the fact that CIM-FN was derived by heating cells at 90 °C for 15 minutes. This observation suggests that the activating target is thermally stable. This was confirmed by the data shown in Figure 3B where RA (relative activity) was measured after CIM-FN was further treated at 90 °C for 5, 10, 20, 30 and 60 minutes (the activity with no heat treatment was taken as 100). Although CIM-FN further treated at 90 °C showed reduced activity, the remaining activity was still quite high. For instance, CIM-FN treated at 90 °C for 20 minutes still induced 55 % activity when compared to no heat treatment. As noted above, the thermal stability of the target is beneficial for DNAzyme assays where heat can be used to inactivate nucleases (DNases and RNases) that otherwise would interfere with DNAzyme assays (Figure S1).
The kinetics of DT4 was analyzed using single-turnover conditions (E/S=20; Figure S8). DT4 was incubated with concentrated CIM-FN (to saturate the binding site of the DNAzyme) over 12 hours. The observed rate constant (kobs) was determined to be 0.38±0.01 h−1 (Figure S8).
Based on the observation above, it is possible that the DNAzyme activator is a small metabolite as small molecules are in general more stable than macromolecules such as proteins. This hypothesis was refuted based on the test that treating CIM-FN with proteinase K for 1 hour prior to the DNAzyme assay completely abolished the cleavage activity (Figure 3C). This suggests that the target is proteinaceous in nature. Following this, CIM-FN was incubated with a ribonuclease inhibitor (Figure S9) to determine if the cleavage of the RNA linkage was caused by a ribonuclease. There was no difference in cleavage activity when comparing CIM-FN treated with or without the inhibitor, suggesting that the observed RNA cleavage activity was not mediated by a ribonuclease.
Fractionation of CIM-FN with molecular weight cut-off devices suggests that the protein target has a molecular weight of approximately 30,000 to 100,000 Daltons, as cleavage was observed with the filtrates produced from filters with molecular weight cut-offs at 30,000, 50,000 and 100,000 Daltons. However, the most robust cleavage was seen with the 100,000 Dalton filtrate, followed by the 50,000 Dalton filtrate (Figure 3C), suggesting that the molecular weight of the protein target is around 50,000 Daltons. Currently, the exact identity of the molecular target for DT4 is under investigation and the results will be reported in the future.
Next, the specificity of the DNAzyme was examined. The cleavage activity of DT4 was assayed against several anaerobic species commonly found in the oral or gut microbiota, as well as two aerobic bacteria (Figure 4).13 The bacteria are: Bacteroides fragilis (BF), Clostridium difficile (CD), Bacteroides vulgatus (BV), Bacteroides intestinalis (BI), Coprococcus comes (CC), Dorea longicatena (DL), Collinsella aerofaciens (CA), Streptococcus salivarius (SS), Escherichia coli (EC) and Bacillus subtilis (BS). DT4 displayed strong cleavage activity only in response to CIM-FN. The unheated CIM of EC induced minor cleavage, and the CIM of BS produced DNA degradation, but this was likely caused by nucleases, judging by the smearing pattern on the dPAGE. CIMs from the remaining bacteria did not induce any cleavage.
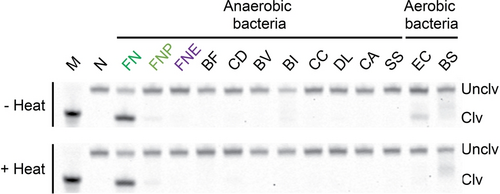
Recognition specificity of DT4. Reactivity of DT4 in the presence of CIM of some common anaerobic bacteria in human gut and oral flora and two common aerobic bacteria. The bacteria include FN ssp. nucleatum (FN), FN ssp. polymorphum (FNP), F. necrophorum (FNE), Bacteroides fragilis (BF); Clostridium difficile (CD), Bacteroides vulgatus (BV), Bacteroides intestinalis (BI), Coprococcus comes (CC), Dorea longicatena (DL), Collinsella aerofaciens (CA), Streptococcus salivarius (SS), Escherichia coli (EC) and Bacillus subtilis (BS). “+Heat”: CIM additionally heated 90 °C for 15 minutes. “-Heat”: CIM without additional heating step. All DNAzyme assays were conducted at 23 °C in 1× SB at E/S=100/1 for 4 hours. M: marker, N: negative control (no reaction control). The uncropped images of the two gels in this figure are provided in Figure S12 (panels E and F, respectively) in the Supporting Information.
In order to eliminate any cross reactivity caused by non-specific cleavage, we took advantage of the heat-resistant property of the activating target for RFD-FN1. The CIMs from all bacteria were heat-treated at 90 °C for 15 minutes and then tested for inducing the cleavage activity of DT4. The heat treatment completely abolished the faint cleavage activity previously exhibited by EC. Even though BS still induced non-specific degradation, it did not induce any cleavage at the RNA site. Overall, these results suggest that the RNA cleavage activity of DT4 is specific for FN.
The bacterium we used for the DNAzyme isolation is FN ssp. nucleatum. We tested the activity of DT4 in response to the CIM of FN ssp. polymorphum (FNP), another subspecies of FN. Interestingly, DT4 showed a detectable but significantly reduced activity with CIM-FNP (Figure 4). It is possible that DT4 is activated by a target present in both subspecies of FN, but that the level of the target is significantly higher in FN ssp. nucleatum.
We also examined the activity of DT4 towards F. necrophorum (FNE), which represents a different species within the same Fusobacterium genus. CIM-FNE did not induce any cleavage activity of DT4 (Figure 4), strongly suggesting that DT4 is FN species-specific.
We investigated the detection sensitivity of DT4. Using dPAGE method, DT4 was able to achieve a limit of detection of 107 CFU/mL (Figure 5A). However, with the incorporation of a culturing step, the detection sensitivity can be greatly improved. Following a culturing time of 24 hours, DT4 was able to detect 100 CFUs. When the culturing time was extended to 36 hours, the assay was able to detect a single CFU (Figure 5B). The two gold standards for FN detection are cell culture and PCR; the limit of detection (LOD) of these two methods has been reported to be 103–104 CFUs and are 101–102 CFUs, respectively.4 Therefore, with a 36-hour culturing step, the DNAzyme system is capable of delivering a superior LOD.
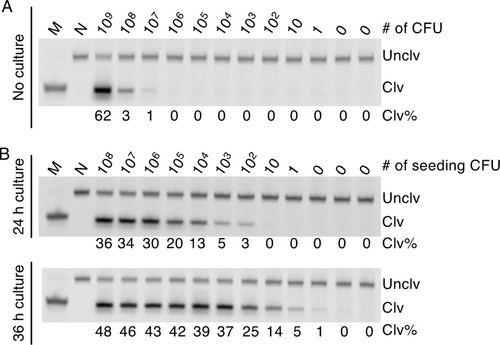
Detection sensitivity of DT4. Limit of detection (LOD; CFU/mL) of DT4 without (A) and with cultivation (B) for 24 and 36 h. All DNAzyme assays were conducted at 23 °C in 1× SB at E/S=100/1 for 4 hours. M: marker, N: negative control (no reaction control). The uncropped images of the three gels in this figure are provided in Figure S12 (panels G, H and I, respectively) in the Supporting Information.
We also examined the functionality of DT4 in human saliva and stool. For saliva testing, a pooled saliva sample (Innovative Research, Michigan, USA) and CIM-FN prepared from 109 FN cells were heated at 90 °C for 15 minutes, and then spiked with CIM-FN in 1× SB. This was followed by the addition of DT4. The concentration of saliva was examined at 0 %, 5 %, 10 %, 20 %, 30 %, 40 %, and 50 % (v/v). The DNAzyme activity was tested using the dPAGE protocol discussed earlier. The data presented in Figure S10 indicates that DT4 is fully functional in human saliva.
Furthermore, a real-time fluorescent assay with DT4 (Figure 6) was performed by heat treating the CIM-FN and pooled saliva as described above. CIM-FN was concentrated and diluted with 1× SB to achieve the cell counts of 2.3×108, 1.2×108, 4.6×107, 2.3×107, and 2.3×106 CFU/mL. CIM-FN at each cell count was then spiked into saliva. DT4 was able to produce time-dependent fluorescence signal increase with CIM-FN prepared from as low as 2.3×107 cells, demonstrating fluorescence-based detection of FN in saliva.
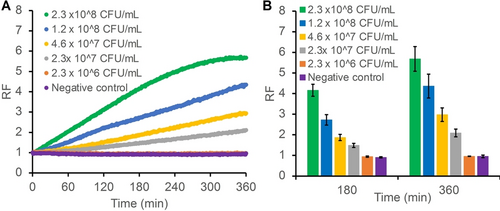
(A) Real-time fluorescent assay with DT4 and CIM-FN spiked saliva. CIM-FN was concentrated 10× using a 30 kD centrifugal device and then diluted with 1× SB to achieve the cell counts reported above. Relative fluorescence (RF) is represented as F/F0, where F is fluorescence at each respective time point and F0 is the fluorescence at the time when CIM-FN was added. (B) RF values at 180-minute and 360-minute time points. The real-time fluorescent assay with DT4 and CIM-FN spiked saliva was performed in duplicates and the RF values at 180 and 360 minutes were calculated and plotted. The error bars represent standard deviation.
For the experiment with the human stool, we first prepared a solubilized stool sample from pooled crude human stools. The solubilization was done via homogenization of 100 mg crude solid stool with 1 mL of 1× SB. After centrifugation to pellet undissolved materials, the supernatant was collected and heated at 90 °C for 15 minutes. The as-prepared solubilized stool was then spiked with heat-treated (90 °C for 15 minutes) CIM-FN in 1× SB prepared from 109 FN cells, followed by the addition of DT4. The concentration of the solubilized stool was examined at 0 %, 5 %, 10 %, 20 %, 30 %, 40 % (v/v). The dPAGE analysis was conducted to analyze the DNAzyme activity. In contrast to the lack of notable effect observed with human saliva, the activity of DT4 was reduced with increasing amounts of solubilized human stool (Figure S11). In 20 % stool, the relative activity of the DNAzyme was reduced by ~62 %; when the stool fraction was increased to 40 %, the relative activity was decreased by 74 % (26 % relative activity remained). These observations indicate that human stool represents a more complex sample matrix and contains molecules that inhibit the activity of DT4. Nevertheless, DT4 remains partially functional in solubilized human stool.
Conclusion
In summary, using in vitro selection, we have discovered a cis-acting RNA-cleaving DNAzyme (named RFD-FN1) as well as a significantly truncated trans-acting version (named DT4) that can be activated by a target produced by Fusobacterium nucleatum ssp. nucleatum. This target is also present in Fusobacterium nucleatum ssp. polymorphum but absent in Fusobacterium necrophorum, a different Fusobacterium species. These results demonstrate that the new DNAzyme is Fusobacterium nucleatum subspecies selective. The DNAzyme has a unique sequence feature that has not been observed with previously reported DNAzymes: its proposed catalytic core is highly rich in A and T (91.7 %). Intriguingly, the target that activates the DNAzyme is greatly resistant to heat denaturation: ~50 % activity remains after heating at 90 °C for 20 minutes. This heat-stable property of the target is advantageous for specificity consideration as heat can be used to denature both DNases and RNases, which are ubiquitous in all types of biological samples and can degrade nucleic acid probes. Our work also shows that heat denaturation of complex biological sample in combination with in vitro selection can lead to the discovery of new and unique biomarkers. Another intriguing finding from this work is that it may not be necessary to use unintended bacterial species in a counter-selection step prior to the positive selection with an intended bacterium, an assumption that was made in our previous studies for the consideration of eliminating DNAzymes with cross-activities from the selection process. Even though we did not include a counter-selection step, we were still able to derive a DNAzyme that is highly specific for FN. However, it is possible that the heat denaturation step may have narrowed down the target range within CEM-FN as the harsh heat treatment should have denatured most of the protein targets within CEM-FN, making them unsuitable as the targets for the DNAzyme selection. Finally, the discovery of a new, highly specific DNAzyme probe for Fusobacterium nucleatum creates an opportunity to develop simple and accurate screening tests against this pathogen that is associated with multiple human diseases, an interest our group is actively pursuing.
Acknowledgments
This work was supported by grants from Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (Y.L.) and the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI) and the Ministry for Research and Innovation (MRI) of Ontario.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




