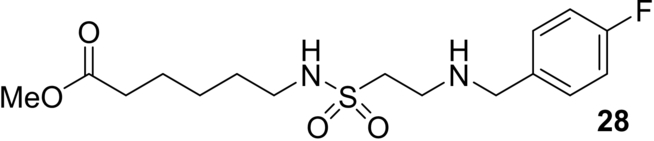
Ring Expansion Strategies for the Synthesis of Medium Sized Ring and Macrocyclic Sulfonamides
Abstract
Two new ring expansion strategies are reported for the synthesis of medium sized ring and macrocyclic sulfonamides. Both methods can be performed without using classical protecting groups, with the key ring expansion step initiated by nitro reduction and amine conjugate addition respectively. Each method can be used to make diversely functionalised cyclic sulfonamides in good to excellent yields, in a range of ring sizes. The ring size dependency of the synthetic reactions is in good agreement with the outcomes modelled by Density Functional Theory calculations.
Introduction
Sulfonamides are amongst the most important structural motifs in medicinal chemistry, being widely present in FDA approved small molecule drugs with broad spectrum biological activity.1, 2 Cyclic sulfonamides—traditionally known as “sultams”—are also commonly found in drugs and bioactive compounds, with representative examples of both classes shown in Figure 1.3

Medicinally important acyclic and cyclic sulfonamides.
Compared to normal sized ring cyclic sulfonamides (5–7-membered rings), medium sized (8–11-membered) and macrocyclic (12+ membered, e.g. 7) sulfonamides are far less well explored, both in terms of synthetic methods to prepare them, and their biological activities.4, 5 Indeed, this mirrors a trend seen across other compound classes, where larger ring systems are often underexplored due the additional challenges associated with their synthesis.6, 7 Given growing interest in the study of medium sized rings and macrocycles in medicinal chemistry,8, 9 the development of effective new methods to prepare them is important, especially for compounds classes like sulfonamides for which existing synthetic methods are limited.
This manuscript is focused on the development of two new methods to prepare medium sized and macrocyclic sulfonamides using ring expansion reactions.10, 11 Ring expansion reactions offer significant advantages for large ring syntheses, most notably by removing the need to perform an end-to-end cyclisation via a medium sized ring or macrocyclic transition state. Our group has experience designing new ring expansion methods for large ring synthesis, generally using a sequence of acylation, protecting group cleavage and ring expansion to make lactams and lactones of the type summarised in Scheme 1A (8→9c).12 However, we had not reported any sulfonamide-forming ring expansion reactions prior to this study. Furthermore, to the best of our knowledge, we are aware of only one other published study in which a ring expansion reaction of any type has been used to prepare a medium sized or macrocyclic sulfonamide.13
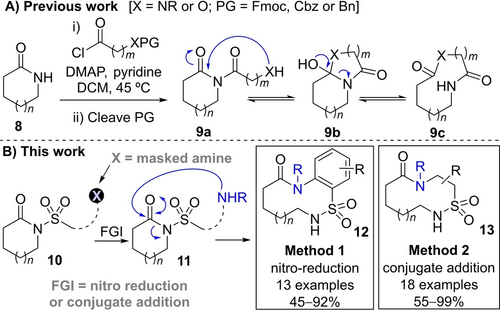
A) Previous work; B) Ring expansion strategies for the synthesis of medium sized and macrocyclic sulfonamides.
Two new sulfonamide-forming ring expansion protocols are reported herein. Both rely on the use of sulfonylated precursors of the form 10, which contain a group “X” that can be converted into an amine (10→11) capable of initiating ring expansion (11→12/13, Scheme 1B). In contrast to our previous work,12 in this study we were keen to avoid the use of classical protecting group chemistry, and have developed complementary strategies to achieve this using redox chemistry (nitro reduction, Method 1) and amine coupling (conjugate addition, Method 2) respectively. Both methods can be used to make diversely functionalised cyclic sulfonamides in good to excellent yields, and work well across a range of ring sizes, with the ring size dependency of the synthetic reactions in good agreement with the outcomes predicted by Density Functional Theory (DFT) calculations.
Results and Discussion
Our reaction design strategies are summarised in Scheme 2. The first method (Scheme 2A, Method 1) is based on the reduction of 2-nitro sulfonamide precursors of the form 14; reduction of the nitro group was expected to form aniline 15RO, and this ring-opened form was predicted to rearrange via cyclisation to form ring closed isomer 15RC and subsequent ring expansion to form 15RE. This approach allows access to benzannulated products and has the added advantage that the 2-nitro sulfonamide moiety in the starting material can be easily introduced by reacting lactams with commercially available 2-nosyl chloride. To access non-benzannulated systems an alternative reaction design was envisioned, starting from vinyl sulfonamide precursors of the form 16 (Scheme 2B, Method 2); in this case, a conjugate addition reaction between 16 and an added primary amine was proposed, to set up a system capable of undergoing cyclisation (17RO→17RC) and ring expansion (17RC→17RE) in the same way.
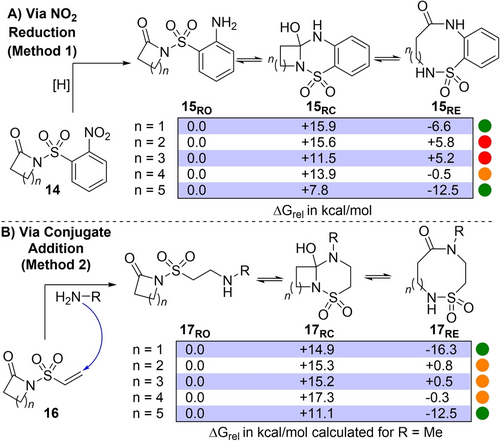
Reaction design and DFT calculations to predict the influence of ring size on reaction viability. Relative energies are Gibbs energies at the B3LYP/6-31G* level of theory in the gas phase, at 298.15 K.
Before embarking on synthetic studies, the viability of both proposed ring expansion reactions was assessed by DFT calculations, using methodology validated and benchmarked with imide-based systems during our previous ring expansion work.12e For both reaction series, the ground state energies of the ring-opened (15/17RO), ring-closed (15/17RC) and ring-expanded (15/17RE) isomers were calculated; our previous work shows that the isomer calculated to be lowest in energy typically aligns well to synthetic outcomes. As the ring-size of the starting material is known to have a major influence on the outcomes of ring expansion reactions, these calculations were performed for starting material ring sizes ranging from 4–8-membered (14/16, n=1–5), with the results summarised in Scheme 2. A traffic light colour coding system is depicted, with green indicating a reaction calculated to be thermodynamically viable, red indicating a reaction calculated to be not viable, and amber for borderline cases.
For the nitro reduction series (14→15, Scheme 2A), a clear thermodynamic driving force for ring expansion was revealed for 4- and 8-membered starting materials (14, n=1 and 5), indicated by the much lower relative energy of the isomer 15RE. In contrast, the expansion of 5- and 6-membered starting materials (14, n=2 and 3) were predicted to be not viable; this is not wholly surprising, as “normal-to-medium” ring expansions of this type are usually the most challenging.10d The ring expansion of the 7-membered starting material (14, n=4) was calculated to be a borderline case. For the conjugate addition series (16→17, Scheme 2B), a clear driving force for expansion was again seen for the 4- and 8-membered starting materials (16, n=1 and 5). The calculated energies for 17RO and 17RE for the 5–7-membered systems (16, n=2–4) were too close to make a confident prediction on the most likely synthetic outcome, but notably they were all sufficiently encouraging to justify testing synthetically (vide infra).
Synthetic studies started by examining the nitro-reduction series (Method 1). Guided by the DFT results, we started by testing the expansion of 8-membered lactam 18 a, as the ring expansion was calculated to be highly exergonic for this ring size. Conditions were established whereby lactam 18 a could be sulfonylated using n-BuLi and 2-nosyl chloride 19 a to form 20 a in 86 % yield (see Supporting Information for optimisation). Reduction of the nitro group was then done via hydrogenation with Pd/C in ethyl acetate, and the resulting aniline was converted directly into the ring-expanded macrocyclic sulfonamide 21 a following RT stirring with DBU in CH2Cl2, in 90 % overall yield over the reduction/ring expansion sequence (Scheme 3A).
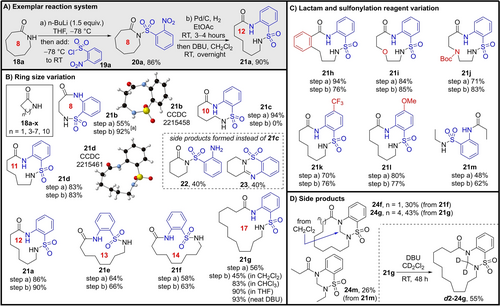
Ring expansion method for the synthesis of medium sized and macrocyclic sulfonamides initiated via nitro reduction (Method 1). All examples were performed using the conditions summarised in (A) (see Supporting Information for full synthetic details) and yields are of isolated product.[a] In this example the ring expansion took place spontaneously following hydrogenation, so the DBU step was not required.
With reaction conditions established, the scope of the reaction was next examined, starting with the effect of ring size (Scheme 3B). As predicted by the DFT calculations, the expansion of β-lactam 18 b to form 8-membered ring sulfonamide 21 b worked well, with the reduction/ring expansion step proceeding in 92 % yield. The DFT calculations also correctly predicted that the expansion of 6-membered valerolactam would fail; none of the ring expanded product 21 c was formed, with aniline 22 and condensation side product 23 the only tractable products isolated from the reaction. Pleasingly, all >6-membered ring systems tested worked well, with ring expanded sulfonamides 21 d–g all formed in good yields. Variation to the lactam precursor (to form 21 h–j) and sulfonylation reagent (to form 21 k–l) was also well tolerated, enabling the synthesis of more functionalised derivatives using the same method (Scheme 3C). Interestingly, the method is also applicable to linear amide precursors; for example, linear sulfonamide 21 m was prepared using the same sequence, starting from N-ethylpropanamide, via the overall insertion the 2-amino sulfonyl benzene unit highlighted in blue.
Notably, in cases where the yield of the ring expansion products was lower than in ideal systems, the bulk of the mass balance accounted for by the formation of methylene-bridged side products (e.g. 24 f/g, 24 m Scheme 3D). These products presumably arise from an unusual coupling of the ring-expanded cyclic sulfonamide product with CH2Cl2 (the solvent used for the ring expansion step). This notion was corroborated by the fact that deuterated analogue d2–24 g was formed upon stirring 21 g with DBU in CD2Cl2.14 Whilst not intended, this unexpected side reaction allows 1,2,4-thiadiazinane 1,1-dioxides to be incorporated into the macrocyclic products. This may prove to be a useful discovery, given that these motifs are present in a range of bioactive compounds.15 With respect to the desired ring expansion, this side reaction can easily be avoided simply by changing the reaction solvent; for example, product 21 g was formed in a modest 46 % yield when the ring expansion step was performed in CH2Cl2, but the yield increased markedly when done in CHCl3, THF or neat DBU (80 %, 90 % and 93 % yields respectively).
The use of hydrogenation to reduce the nitro group presumably precludes the inclusion of hydrogenation-sensitive groups like alkenes or benzyl alcohols in the starting materials. We therefore briefly examined alternative reduction conditions that do not require hydrogenation, and found that reacting 20 a with zinc and ammonium chloride in a water/methanol mixture enabled reduction and ring expansion to form 21 a in modest (29 %) unoptimised yield. Attempts to reduce 20 a using SnCl2⋅2 H2O and HCl were unsuccessful (for full synthetic details of both alternative methods, see Supporting Information).
We also briefly explored the possibility using ring-expended product 21 a in a second iteration of the sulfonylation/ring expansion sequence.12 Unfortunately, attempts to N-sulfonylate 21 a with 19 a on its lactam moiety failed, with N-sulfonylation of the sulfonamide nitrogen taking place instead. Similar reaction via the sulfonamide nitrogen was also observed when 21 a was reacted with an acid chloride (see Supporting Information for full synthetic details and characterization data for the products formed in each case).
Of final note, several of the products in this series are crystalline solids, and the structures of two of these (21 b and 21 d) are supported by X-ray crystallographic data.16
Next, attention turned to examining the conjugate addition/ring expansion reaction series (Method 2). The requisite sulfonylated lactam starting materials 16 a–e were prepared using two different approaches (Scheme 4A); either the parent lactam was reacted with 2-chloroethanesulfonyl chloride under basic conditions, or they were made via cyclisation of N-sulfonylated amino acid derivatives of the form 25 (see Supporting Information for full synthetic details).17
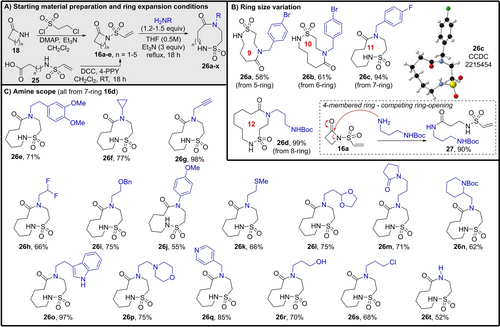
Ring expansion method for the synthesis of medium sized and macrocyclic sulfonamides initiated via conjugate addition (Method 2). All examples were performed using the conditions summarised in (A) (see Supporting Information for full synthetic details) and yields are of isolated product.
The effect of variation in the ring size of the starting material was then explored. The DFT results (vide supra) suggested that 4–8-membered ring precursors 16 a–e could all be viable substrates for this process. Pleasingly, this proved to be the case for 5–8-membered starting materials 16 b–e; simply heating the sulfonylated lactam with the appropriate primary amine and triethylamine in THF enabled smooth conversion into the expected ring expanded 9–12-membered cyclic sulfonamides 26 a–d in good to excellent yields (Scheme 4B). The only substrate that did not react as expected was 4-membered lactam derivative 16 a (see Scheme 4B box). In this case, the ring expansion was calculated to be highly exergonic using our DFT method. However, the DFT method does not account for the possibility of side products being formed if more kinetically favourable pathways are available. In this instance, ring-opening of the strained 4-memebred ring, presumably via the mechanism shown in the Scheme 4B box, was found to be the dominant reaction pathway, leading to the formation of linear amide 27 in 90 % yield. A similar side reaction was also observed when the synthesis of 26 c was attempted using methanol as the reaction solvent rather than THF.18 These results highlight the delicate balance required to achieve the desired reactivity when using starting materials of the type 16, which contain more than one electrophilic site.
Notwithstanding the 4-membered ring system, the ability to perform the conjugate addition/ring expansion method on all of the other ring sizes tested is notable, especially as it works well to promote challenging “normal-to-medium” ring expansions.10d Furthermore, another key feature of this approach is the freedom with which the primary amine component can be varied. Of the 20 examples shown in Scheme 3B and C, 19 different diversely functionalised primary amines were used,19 and in choosing these amines, we actively sought to challenge the method with varied chemical functionality.20 For example, in addition to comparatively simple aliphatic amines (e.g. 26 a–c, 26 e, f), ring-expanded sulfonamides have been generated in good to excellent yields from an amine bearing a terminal alkyne (26 g), a fluorinated amine (26 h), a sulfide-tethered amine (26 k), an aniline derivative (26 j), amines containing protected alcohols, aldehydes and amines (26 d, 26 i, 26 l, 26 n), amines tethered to aza-heterocycles (26 m–q), a free amino alcohol (26 r) and an alkyl chloride (26 s). The conjugate addition/ring expansion cascade also works using ammonia (added as a solution in 1,4-dioxane) to form secondary lactam 26 t. The freedom to introduce varied functionality into the ring expanded products from a common precursor, using a simple and robust procedure,21 is likely to be useful when preparing compound libraries for bioassay or structure activity relationship (SAR) studies.
Conclusion
In summary, two complementary protecting group-free ring expansion methods have been developed for the synthesis of medium sized ring and macrocyclic sulfonamides. The first (Method 1) is initiated via simple nitro reduction, and benefits from the fact that the starting materials can be easily prepared from commercially available 2-nosyl chloride. The second (Method 2) is initiated by conjugate addition with a diverse array of functionalised primary amines. Both new methods work on a range of ring sizes, with the ring size dependence of both synthetic series in good agreement with the reaction outcomes predicted by DFT calculations. Established methods to make medium sized ring and macrocyclic sulfonamides are relatively rare, particularly so for methods involving ring expansion. Therefore, our hope is that the two new methods reported herein will help expedite the exploration of this compound class in a range of applied fields, especially in medicinal chemistry.
Acknowledgments
The authors would like to thank the University of York for the provision of an Eleanor Dodson Fellowship (to W.P.U.) and the China Scholarship Council for a funding the PhD studentship of Z.Y. We are also grateful to Dr Tony Wild for supporting I.Z. through the University of York Wild Fund. J.M.L. is funded through a Royal Society Industrial Research fellowship. The computational work in this project was undertaken on the Viking Cluster, a high-performance computing facility provided by the University of York. We are grateful for computational support from the University of York High Performance Computing service, Viking and the Research Computing team. Finally, we would like to thank the reviewers, whose insightful ideas and suggestions during the peer review process improved this manuscript markedly.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.