Selective Separation of Lithium, Magnesium and Calcium using 4-Phosphoryl Pyrazolones as pH-Regulated Receptors
Abstract
Ensuring continuous and sustainable lithium supply requires the development of highly efficient separation processes such as LLE (liquid-liquid extraction) for both primary sources and certain waste streams. In this work, 4-phosphoryl pyrazolones are used in an efficient pH-controlled stepwise separation of Li+ from Ca2+, Mg2+, Na+ and K+. The factors affecting LLE process, such as the substitution pattern of the extractant, diluent/water distribution, co-ligand, pH, and speciation of the metal complexes involved, were systematically investigated. The maximum extraction efficiency of Li+ at pH 6.0 was 94 % when Mg2+ and Ca2+ were previously separated at pH<5.0, proving that the separation of these ions is possible by simply modulating the pH of the aqueous phase. Our study points a way to separation of lithium from acid brine or from spent lithium ion battery leaching solutions, which supports the future supply of lithium in a more environmentally friendly and sustainable manner.
Lithium has been widely employed in many commercial areas, such as for the manufacture of lithium ion batteries (LIBs), ceramics and glass, lubricating greases, polymers and pharmaceuticals.1 In particular, the demand for LIBs has rapidly accelerated in recent years. A conservative estimation predicts that lithium demand for electric vehicle (EV) batteries will increase by a factor of 17–21 from 36 000 t in 2020 to 620 000–770 000 t in 2050, resulting in a rise in cumulative lithium demand of 18 300 000 t.2 Thus, for a continuous lithium supply to be maintained, it is increasingly important to develop highly efficient separation processes to provide sustainable lithium production in the long term. Currently, salt lake brines are one of the most important lithium sources and more than 59 % of the current lithium reserves are deposited in brines.1c In addition to this high abundance, easy operation and cost-effective advantages also make brines an increasingly important and appealing source for lithium production. Nevertheless, brines generally have a high concentration of co-existing divalent cations such as Mg2+, Ca2+ as well as the monovalent cations Na+ and K+.3 Further, Mg2+ exhibits a very similar ionic hydration radius as well as chemical properties to Li+ (reflecting their diagonal relationship in the Periodic Table), which results in the separation of Li+ from Mg2+ being extremely challenging.4 Liquid-liquid extraction is an attractive process for the separation of lithium due to its generally low cost, high efficiency and large processing capacity.5
Previous studies using tributylphosphate (TBP) and FeCl3 have demonstrated the selective extraction of Li+ from an aqueous solution containing Mg2+ in high concentration,6 but the formation of a third phase, and a problem with dissociation of Li+ after loading, hinders its industrial application potential.7 Selected macrocyclic receptors, including some crown ether-based reagents, have been widely employed for the selective recognition of Li+ since they often exhibit good selectivity for Li+ over Na+ and K+,8 but the effective extraction of Li+ from aqueous phases in the presence of Mg2+, Ca2+ has been less explored and still remains a challenge.3a, 9 In a previous study we investigated a series of 4-phosphoryl pyrazolone ligands bearing an adjustable bite size between chelating O-donor atoms that bind to Li+.10 While we were able to examine in detail the Li+ coordination behavior in the presence of the industrially used co-ligands, trioctylphosphine oxide (TOPO) and tributylphosphate (TBP), as well as to demonstrate their high potential for the selective recognition and extraction of Li+ from alkali metals, their coordination behavior towards divalent Mg2+ and Ca2+ and their potential use for the separation of Li+ from these metal ions remained unexplored.
As an extension of this previous study, we have synthesized a series of new 4-phosphoryl pyrazolone ligands with electron-neutral (R=H, HL1), electron-donating (R=Me, HL2) or electron-withdrawing (R=Cl, HL3; R=CF3, HL4) substituents at each of their phenyl para positions (Figure 1), as it is known that the acid dissociation properties of these ligand types are highly influenced by the electronic nature of such substituents.11 This, in turn, will influence their extraction performance.12 In the present study, the coordination behavior of HL1–HL4 towards Li+, Mg2+ and Ca2+ was studied and experiments evaluating their use for the selective extraction of Li+ from other alkali and alkaline earth metals were conducted. In these experiments, n-octane was used as the organic phase because of the high solubility of the present ligands in this nonpolar solvent (in contrast to the nitro-substituted ligands employed in our previous study).10
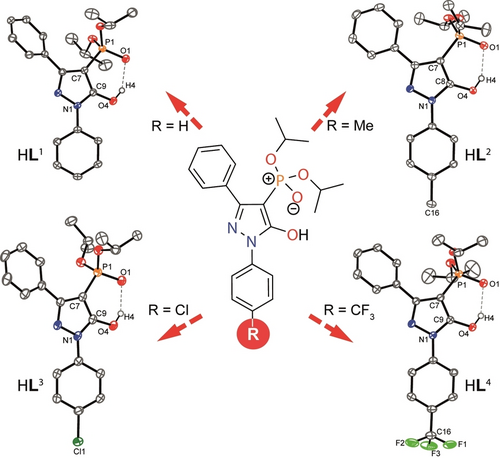
Molecular structures of the ligands HL1–HL4 studied in this work (all carbon hydrogen atoms are omitted for clarity and ellipsoids are drawn at 50 % probability level).
HL1–HL4 were synthesized using our previously reported three-step procedure with some modification (Scheme S1).10, 13 The crystal structures of all four ligands are displayed in Figure 1; each ligand was found to crystallize in its enol-form. The O⋅⋅⋅O separation between the two potentially chelating O-donor atoms, determined from the molecular structures of the respective tetrabutylammonium salts, ([TBA]Ln, n=1–4; Figure S41), are very similar at 3.1315(14)–3.139(2) Å (see Table S1). Hence, as expected, no significant variation of the bite size between ligands is observed. Reaction of HL3 and HL4 with Mg(OH)2 or Ca(OH)2 led to the isolation of the dinuclear complexes [Mg2(L3)4(H2O)2] (3), [Mg2(L4)4(H2O)2]⋅2 CH3CN (4) and [Ca2(L4)4(H2O)2]⋅CH3CN (5).
Single crystals suitable for X-ray diffraction of all three products were obtained either by slow evaporation of the reaction solvent or by slow diffusion of Et2O into a solution of the respective crude complexes.13 In all three cases (see Figures 2 and S42), each metal center (Mg2+ or Ca2+) was shown to have a coordination number of six, involving two six-membered chelate rings formed from the O2-donor set of two deprotonated HL3 or HL4 ligands, one bridging O-donor and one H2O ligand. The two bridging (pyrazolone) O-donors linking both metal centers form a M2O2 core in each complex. The M−O bond lengths involving the phosphoryl units [2.0674(11) Å for 3; 2.068(3) Å for 4; 2.2962(9) Å for 5] for each bridging ligand are shorter than those to the pyrazolone oxygen atoms [2.0898(10) Å for 3; 2.121(2) Å for 4; 2.3723(9) Å for 5]; however, this behavior is reversed for the other two coordinated ligands [2.0072(11) vs. 1.9978(11) Å for 3; 2.019(3) vs. 2.009(2) Å for 4; 2.261(1) vs. 2.2478(10) Å for 5].
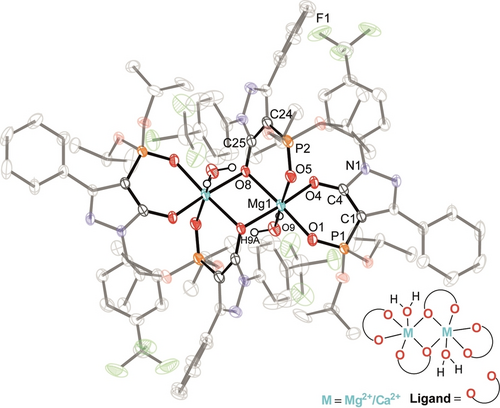
Molecular structure of [Mg2(L4)4(H2O)2]⋅2 CH3CN (4) (all carbon hydrogen atoms and CH3CN solvent are omitted for clarity and ellipsoids are drawn at 50 % probability level).
The coordination behavior of the above ligands with Li+, Mg2+ or Ca2+ in the presence of industrially important trioctylphosphine oxide (TOPO, as co-ligand) was investigated in order to evaluate the potential of such mixed-ligand systems for achieving selective metal-ion binding and phase transfer via a liquid-liquid extraction process. Accordingly, in an initial experiment we reacted HL4 with Mg(OH)2 in CH2Cl2 in the presence of TOPO (HL4 : Mg(OH)2 : TOPO=1 : 1:0.5) which led to the formation and isolation of complex 613 whose crystal structure is shown in Figure 3a.
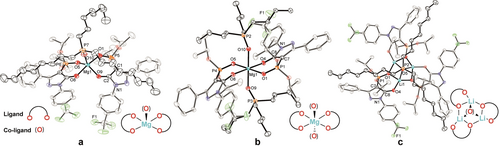
Molecular structures of a) [Mg(L4)2(TOPO)] (6), b) [Mg(L4)2(TBPO)2] (7), c) [Li3(L4)3(TOPO)] (9), including schematic representations of their respective coordination modes (hydrogen atoms are omitted for clarity and ellipsoids are drawn at 50 % probability level).
The effect of a higher concentration of TOPO on the coordination behavior was also studied. Thus, the reaction of HL4 with Mg(OH)2 in CH2Cl2 in the presence of 1.0 equiv of TOPO was investigated, however, attempts to crystallize a corresponding Mg2+ complex were unsuccessful.13 This can be rationalized in terms of the high solubility of the product in CH2Cl2 and this high solubility remained even when nonpolar pentane or heptane was employed for the reaction. In view of this, reaction of 4 with the co-ligand tributylphosphine oxide (TBPO) (4 : TBPO=1 : 4) incorporating shorter carbon chains was carried out in heptane.13 In this case single crystals of [Mg(L4)2(TBPO)2] (7) suitable for X-ray analysis were successfully obtained by slow evaporation of the heptane solution. The crystal structure of 7 is shown in Figure 3b. The complex has a stoichiometry of Mg2+ : [L4]− : TBPO of 1 : 2 : 2 and an octahedral coordination geometry with Mg1−O9=2.0804(12) Å and Mg1−O10=2.0673(12) Å. Reactions of HL1 and HL4 with LiOH⋅H2O in CH2Cl2 in the presence of TOPO gave the trinuclear complexes [Li3(L1)3(TOPO)] (8) and [Li3(L4)3(TOPO)] (9). Each of these products incorporate similar binding modes to those observed in our previous study (Figures 3c and S43).10
Some β-diketones, such as 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one, benzoyl-1,1,1-trifluoroacetone and the newer Cyanex 936P (Solvay) reagent are effective and commonly employed for the extraction of lithium.14 For some reagents, a very basic aqueous solution (pH 10–11) is generally necessary.14b-14d However, considering the widespread use of neutral or acidic brines as a lithium source,15 it is increasingly desirable from a cost saving and environmental perspective to employ a ligand class that can be used in neutral or even acidic conditions. Thus, we investigated the extraction ability of HL1–HL4 for Li+ in n-octane by LLE experiments (at pH 5). The control experiments indicate that the presence of TOPO is indispensable for substantial aqueous/organic phase transfer of Li+, Mg2+ and Ca2+ to occur.13 Hence, all LLE experiments in the present study were carried out in the presence of TOPO. In these studies, the highest Li+ extraction (77 %) was observed for HL4 while the lowest extraction of this ion (23 %) was observed for HL2 (Figure 4a). The results confirm that the electronic properties of the substituents located at the para-phenyl position in HL1–HL4 have a considerable influence on extraction performance. This can be accounted for in terms of the respective acid dissociation differences due to the different inductive effects of the substituents.11 Compared with neutral (R=H) and electron-donating (R=Me) groups, electron-withdrawing (R=Cl, CF3) groups will be reflected by weaker O−H bonds.16 Thus, the weakest O−H bond occurs in HL4 because the trifluoromethyl (−CF3) substituent has the strongest inductive (−I) effect. Consequentially, the highest extraction occurs for HL4.
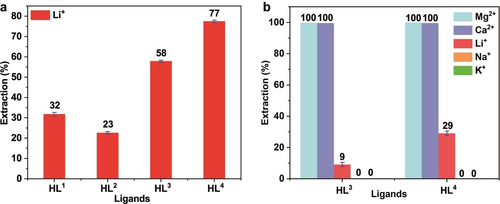
a) Percent extraction of Li+ with different ligands in the presence of TOPO. Conditions: [LiCl]=0.01 M, [NH4Cl]=0.1 M, pH 5±0.2 (HEPES buffer), [HL]=0.1 M, [TOPO]=0.2 M in n-octane, 298 K, 1 h; b) Percent extraction of Li+/Na+/K+/Mg2+/Ca2+ with HL3 and HL4 in presence of TOPO. [LiCl]=[NaCl]=[KCl]=[MgCl2]=[CaCl2]=0.01 M, [NH4Cl]=0.1 M, pH 4.2 (HEPES buffer), [HL]=0.1 M, [TOPO]=0.2 M in n-octane, 298 K, 1 h.
To test their reusability in the LLE process, the best-performing 4-phosphoryl pyrazolone ligand HL4 was employed in a reuse assay of four cycles. After Li+ extraction at pH 5, a 0.5 M HCl aqueous solution was used to strip Li+ from the loaded organic phase (V(aq) : V(org)=1 : 1) and the resulting organic solution was used for another Li+ extraction. The Li+ concentration of the aqueous phase before and after each extraction was determined by ICP-OES analysis and revealed a Li+ extraction of 77 to 78 % for each extraction cycle (Figure S44). Furthermore, stripping of 100 % was achieved in each cycle (Table S3) demonstrating the ease of recovery of the extracted metal ion and the organic phase by acid treatment.
To gain information on the relative lipophilicities of the ligands, which will clearly play an important role in influencing the latters’ extraction behavior, the n-octanol/water distribution was determined in each case (see Supporting Information for details).13 An occurrence of ≈90 % transfer to the organic phase was determined for all ligands except HL1 (for which a value of ≈80 % was obtained). From these results, no major difference in extraction ability related to HL1–HL4 ligand lipophilicities is expected. To check a potential bleeding of the ligand in the LLE experiments, UV/Vis spectra of the aqueous and organic phases of the reuse assay were recorded. While in the samples of the aqueous phases only traces adsorption are detected, the absorption maximum at about 273 nm of the organic phase samples varies by values of 0.5 (Figure S45) indicating that there was no obvious ligand leakage into the aqueous phase during the reuse assay. Presumably the presence of highly lipophilic co-ligand TOPO prevents the leakage of the formed complex in the extraction process.
In order to investigate the selectivity for Li+, competitive LLE experiments in the presence of equimolar concentrations of Na+, K+, Mg2+ and Ca2+ were carried out employing the higher performing ligands, HL3 and HL4. The results indicated that 100 % of both Mg2+ and Ca2+ were extracted while only 9 % (for HL3) and 29 % (for HL4) of the Li+ present were extracted; no extraction of Na+ or K+ was observed (Figure 4b). Clearly, under the conditions employed, both HL3 and HL4 exhibit excellent extraction ability for Mg2+ and Ca2+ (at the equilibrium pH of 4.2) while the extraction of Li+ is limited - presumably due to the competition from the Mg2+ and Ca2+.
In order to investigate solution speciation, slope analyses were carried out for the Mg2+, Ca2+ and Li+ species. The LLE reaction equation is given in Equation (S4)–(S6) and the corresponding logD–pH, logD–log[HL](org), logD–log[TOPO](org) plots are shown in Figures 5 and S46. The determined slopes are approximately 2 for Mg2+ and Ca2+ and 1 for Li+. These results are in accord with the composition of the divalent cation species in solution being [M(L)2(TOPO)2] (M=Mg2+ or Ca2+); it thus seems likely that these complexes have a similar structure to that determined for 7 in the solid state (see Figure 3b).
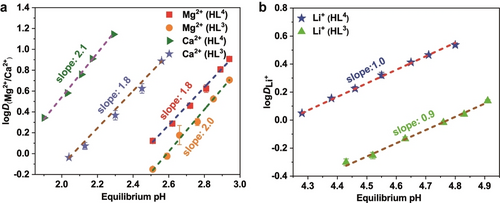
Dependence of the extraction of a) Mg2+, Ca2+ and b) Li+ on the equilibrium pH. Conditions: [LiCl]=[MgCl2]=[CaCl2]=0.01 M, [NH4Cl]=0.1 M, pH 1.9–4.9 (HEPES/HCl buffer), [HL]=0.1 M, [TOPO]=0.2 M in n-octane, 298 K, 1 h.
While the extraction results for Li+ indicate the formation of complexes of stoichiometry 1 : 1 : 1 (Li+ : [L]−:TOPO), it appears likely that the actual composition is 2 : 2 : 2 (Li+ : [L]− : TOPO), that is [Li2(L)2(TOPO)2], which is consistent with the results presented in our previous study.10
From Figure 5, it is noted that the required pH (>4.2) for effective Li+ extraction (that is, >50 %) is significantly higher than observed for the extraction of Mg2+ or Ca2+, which indicated that the selective separation of these three metal ions might be achieved by LLE involving the stepwise regulation of the pH of the aqueous phase. To further test this hypothesis, extraction experiments were performed to evaluate the influence of pH (Figure S47) and showed that complete extraction of Ca2+ and Mg2+ is possible at low pH values. Accordingly, such competitive LLEs employing HL4 were carried out. In the initial experiment an aqueous solution was employed containing equimolar amounts (all 0.01 M) of Li+, Na+, K+, Mg2+ and Ca2+ at an equilibrium pH of 2.0; the results are presented in Figure 6. Under the conditions employed (see caption to Figure 6) extraction of 64 % for Ca2+ occurred while only 18 % of Mg2+ was extracted, with no extraction of Li+, Na+ or K+ occurring. This is in accord with Ca2+ being able to be essentially removed following several reiterations of this first step.
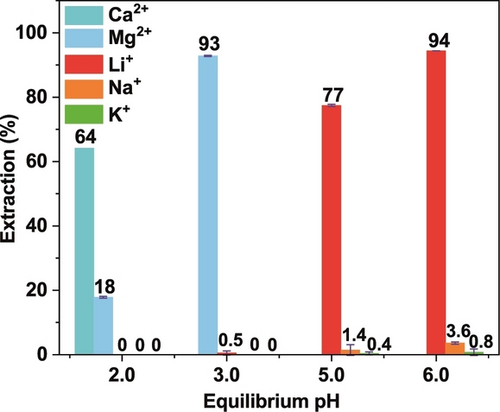
Percent extraction of Ca2+/Mg2+/Li+/Na+/K+ at different pH values. Aqueous phase (HEPES/HCl or Tris/HCl buffer, containing [NH4Cl]=0.1 M in all solution): pH 2.0: [LiCl]=[NaCl]=[KCl]=[MgCl2]=[CaCl2]=0.01 M; pH 3.0: [LiCl]=[NaCl]=[KCl]=[MgCl2]=0.01 M; pH 5.0: [LiCl]=[NaCl]=[KCl]=0.01 M; pH 6.0: [LiCl]=[NaCl]=[KCl]=0.01 M; Organic phase: [HL4]=0.1 M, [TOPO]=0.2 M in n-octane; 298 K, 1 h.
Following from the above result, a second aqueous solution at an equilibrium pH of 3.0 was prepared, this time containing equimolar amounts (at 0.01 M) of Li+, Na+, K+ and Mg2+ (but no Ca2+). In this case, 93 % of Mg2+ was extracted while only 0.5 % of Li+ was co-extracted, with no Na+, K+ extraction being observed. Similarly, a further two solutions were prepared, with both incorporating equimolar amounts of Li+, Na+ and K+ (again each at 0.01 M) but now with equilibrium pH values of 5.0 and 6.0, respectively. For the solution at pH 5.0, 77 % of Li+ was extracted while the value for Na+ was 1.4 % and that for K+ was <1 %. At pH 6.0, the extraction rose to 94 % for Li+ but was only 3.6 % for Na+, with the value for K+ remaining at <1 %. Collectively, the above results demonstrate that by employing our new 4-phosphoryl pyrazolone ligands it is feasible to achieve stepwise LLE separation of Ca2+, Mg2+ and Li+ by pH regulation in an acidic pH range. However, it should be noted that the pH needs to be controlled, since the ligands extract metal cations and release protons to the aqueous phase according to the charge on the extracted cation.17 Thus in the absence of pH control reduced extraction will occur. The pH-controlled selectivity obtained may be explained by the difference in Lewis acidity and hydration energy of the respective metal ions.18 The Lewis acidities of Mg2+ and Ca2+ are apparently higher than that of Li+, and the values decrease in the order Mg2+>Ca2+>Li+,18a suggesting that the divalent cations Mg2+ and Ca2+ have higher binding affinity towards the Lewis basic oxygen-donor atoms of the ligand than the monovalent cation Li+. The hydration energies for Mg2+ and Ca2+ are −1940 kJ mol−1 and −1515 kJ mol−1.18b Thus, the higher hydration energy of Mg2+ compared to Ca2+ will apparently undermine its binding affinity towards oxygen-donor atoms and phase transfer ability from the aqueous to the organic phase.18c This may explain the observed selectivity of Ca2+ over Mg2+ in the LLE process. As mentioned above, the strong electron-withdrawing substituent (−CF3) dramatically weakens the O−H bond in the azole unit, allowing its dissociation under the acidic equilibrium pH and leading to the observed stepwise extraction of Ca2+>Mg2+>Li+ at a low pH.
In summary, herein we report a new series of 4-phosphoryl pyrazolone ligands exhibiting good solubility in nonpolar solvents. Single crystal X-ray analyses demonstrate that diverse binding modes occur in their respective Mg2+, Ca2+ and Li+ complexes which also incorporate TOPO as a co-ligand. Compared to direct removal of Ca2+ and Mg2+,19 the use of a pH-regulated stepwise separation strategy for the present alkali and/or alkaline earth metals is much less common.
We suggest that our study points the way towards industrial applications such as for the separation of lithium from acidic brine or from spent lithium-ion battery leaching solutions20 thus aiding the future supply of lithium in an environmentally friendly and sustainable manner.
Acknowledgments
This work was supported by the Sino-German Collaboration of the German Science foundation (Project No. WE4621/4-1/392417756) and German Federal Ministry for Economic Affaris and Climat Action (SWELL project 03ETE042C). J.Z. thanks the China Scholarship Council (CSC No. 201804910750) for financial support. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




