Efficient Amination of Activated and Non-Activated C(sp3)−H Bonds with a Simple Iron–Phenanthroline Catalyst
Abstract
A readily available catalyst consisting of iron dichloride in combination with 1,10-phenanthroline catalyzes the ring-closing C−H amination of N-benzoyloxyurea to form imidazolidin-2-ones in high yields. The C−H amination reaction is very general and applicable to benzylic, allylic, propargylic, and completely non-activated aliphatic C(sp3)−H bonds, and it also works for C(sp2)−H bonds. The surprisingly simple method can be performed under open flask conditions.
Aspects of sustainability are playing an increasingly important role for the development of new synthetic methods. While it is now generally considered possible to prepare almost any stable molecule of interest with existing methods, current and future challenges shift towards the development of new reactions that make use of benign and abundant reagents and catalysts. For example, the direct conversion of C−H bonds into C-heteroatom bonds has been recognized as a powerful toolbox for designing streamlined and more efficient synthetic routes. Much progress has been made towards C−H functionalization over the past decades.1 However, most reported methods require an activation of the C−H bond such as a neighboring π-system or heteroatom in order to overcome the low reactivity of aliphatic C−H bonds.2 Furthermore, most reported methods rely on catalysis with noble metals such as Rh, Pd, Ir and Ru. Considering economic, environmental as well as health aspects, earth-abundant first row transition metals are more desirable, and especially iron as a non-toxic metal with a high abundance in the Earth's crust.3, 4, 5 However, often used in combination with ligands which need to be synthesized in a multistep fashion, the advantage of this catalysis is often diminished. Thus, despite the enormous progress made over the past decades regarding the development of direct C−H functionalization methodology, issues of practicability and sustainability in combination with the functionalization of aliphatic, non-activated C(sp3)−H bonds remain an important problem (Figure 1 a).
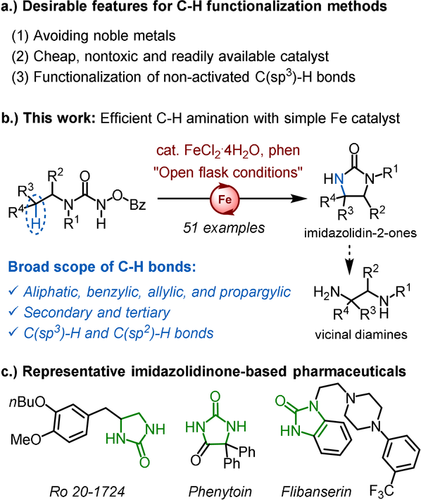
Challenges for C−H functionalization and this work.
Herein, we report a direct C−H amination of benzylic, allylic, propargylic, and completely non-activated aliphatic C(sp3)−H bonds using a simple iron salt in combination with a readily available ligand under mild conditions without the need for inert atmosphere and dry solvents (Figure 1 b). The method is also applicable to C(sp2)−H bonds. Specifically, iron dichloride in combination with 1,10-phenanthroline catalyzes the smooth conversion of N-benzoyloxyurea to imidazolidin-2-ones which are important motifs in bioactive compounds6 (Figure 1 c) and precursors of valuable vicinal diamines.
The transition metal catalyzed insertion of nitrenes into C−H bonds is an attractive method for the amination of C(sp3)−H bonds.7 We became interested in exploring the use of simple iron salts for such reactions and commenced our study by investigating the ring-closing C−H amination of the N-benzoyloxyurea 1 a8, 9 to the cyclic urea 2, a reaction which we recently disclosed with a ruthenium complex as catalyst.10, 11, 12 Encouragingly, we found that FeBr2 (5 mol %) in CH2Cl2 at room temperature afforded the imidazolidin-2-one, although only in a low yield of just 8 % (Table 1, entry 1) (see Tables S1–6 of the Supporting Information for more details). Slightly higher yields of 11 % and 15 % were obtained with Fe(OTf)2 and FeCl2⋅H2O, respectively (entries 2 and 3). We selected FeCl2⋅H2O as the iron salt of choice and next optimized the reaction conditions and found that 1,2-dichloroethane as the solvent at 50 °C resulted in only slightly higher yields of 18 % (entry 4). However, when we increased the catalyst loading from 5 to 10 mol %, the yield increased to 69 % (entry 5). Almost quantitative yield of the cyclic urea 2 was obtained when 1,10-phenanthroline (phen, 10 mol %) and Na2CO3 were added to the reaction (entry 6). Other co-ligands such as terpyridine (entry 7) or neocuproine (entry 8) are inferior. Conveniently, we found that the reaction can be performed in many solvents such as methanol, THF, acetone and ethyl acetate and we selected MeCN as our standard solvent for the remaining study (entry 9). Also notable for practical purposes, the reaction is not sensitive to air and can be performed under open flask conditions (entry 10).
Entry |
Catalyst |
Loading [mol %] |
Ligand[b] |
Base |
Solvent[c] |
T [°C] |
Yield [%][d] |
|---|---|---|---|---|---|---|---|
1 |
FeBr2 |
5 |
none |
none |
CH2Cl2 |
r.t. |
8 |
2 |
Fe(OTf)2 |
5 |
none |
none |
CH2Cl2 |
r.t. |
11 |
3 |
FeCl2⋅4 H2O |
5 |
none |
none |
CH2Cl2 |
r.t. |
15 |
4 |
FeCl2⋅4 H2O |
5 |
none |
none |
DCE |
50 |
18 |
5 |
FeCl2⋅4 H2O |
10 |
none |
none |
DCE |
50 |
69 |
6 |
FeCl2⋅4 H2O |
10 |
phen |
Na2CO3 |
DCE |
50 |
99 |
7 |
FeCl2⋅4 H2O |
10 |
tpy |
Na2CO3 |
DCE |
50 |
82 |
8 |
FeCl2⋅4 H2O |
10 |
neocup |
Na2CO3 |
DCE |
50 |
50 |
9 |
FeCl2⋅4 H2O |
10 |
phen |
Na2CO3 |
MeCN |
50 |
99 |
10[e] |
FeCl2⋅4 H2O |
10 |
phen |
Na2CO3 |
MeCN |
50 |
99 |
11[e,f] |
FeCl3⋅6 H2O |
10 |
phen |
Na2CO3 |
MeCN |
50 |
40 |
12[e,f] |
Fe(OTf)3 |
10 |
phen |
Na2CO3 |
MeCN |
50 |
46 |
- [a] All reactions were performed on 0.1 mmol scale of 1 a in 1 mL of solvent (0.1 M) under nitrogen atmosphere for 14 h unless noted otherwise. [b] phen=1,10-phenanthroline, tpy=2,6-bis(2-pyridyl)pyridine, neocup=neocuproine. [c] DCE=1,2-dichloroethane. [d] All yields refer to the isolated product after column chromatography. [e] Performed under air. [f] Full conversion.
With optimized reaction conditions in hands, we explored the scope of this new iron catalysis employing a broad variety of N-benzoyloxyurea as depicted in Figure 2 (see Figure S1 of the Supporting Information for a variation of the carboxylate leaving group). We started with the C−H amination of benzylic C(sp3)−H bonds (Figure 2 a, imidazolidin-2-ones 2–23) and found that the method tolerates well a large variety of N-substituents at the urea, including different alkyl groups (2–6), a trimethylsilylmethyl (7), and propargyl group (8). Even an unprotected N-H group provided the C−H amination product (9) in high yields of 92 %. Both electron donating and accepting substituents in the para-position of the phenyl moiety were also successfully converted (products 10–16), as well as sterically more demanding meta- and ortho-methyl groups (17 and 18), 1-naphthyl (19) and 2-naphthyl groups (20), heteroaromatic thiophene (21) and even a coordinating pyridine moiety (22). For the majority of the reactions, the isolated yields were above 90 %, including the amination of a tertiary benzylic C−H bond (30). The desymmetrization of a N-benzoyloxyurea derived from 1,3-diphenyl-2-propanamine providing the 4,5-difunctionalized 2-imidazolidinones 23 with two adjacent stereocenters as the trans diastereomer is a notable exception with only a low yield of 32 % for the cyclic urea. Beyond benzylic C−H bonds, this amination method is also applicable to allylic (24–26, 77–81 % yields) and propargylic C−H bonds (27 and 28, 84 and 91 % yields).

Substrate scope. Standard reaction conditions: Substrate (0.20 mmol), FeCl2⋅4 H2O (0.02 mmol), 1,10-phenanthroline (0.04 mmol), Na2CO3 (0.60 mmol), MeCN (2 mL, 0.1 M), 50 °C, 14 h. All yields refer to the isolated product after column chromatography. [a] Performed on 1 mmol scale.
We next turned our attention to C(sp3)−H bonds that are not activated by a neighboring π-system (Figure 2 b). Such non activated C(sp3)−H bonds are notoriously challenging due to their increased C−H bond strength. To our delight, the amination of secondary (32–37) and tertiary (38–42) aliphatic C(sp3)−H bonds occurred efficiently in good to high yields of 68–95 % with just two exceptions (36 in 34 % yield and 43 in 16 % yield).
Site competition experiments shown in Figure 2 c revealed that product mixtures are obtained when more than one C−H group is available. As to be expected, a benzylic position is strongly favored over a non-activated methylene group (44:44′=3.8:1), but only slightly favored over an aliphatic tertiary C−H bond (45:45′=1.1:1). The formation of a 5-membered heterocyclic imidazolidin-2-one is generally favored over a 6-membered heterocyclic pyrimidin-2(1H)-one but the ratio depends of the degree of activation of the C−H bonds. For example, the 6-membered ring formation by amination of a benzylic C−H bond can compete with the 5-membered ring formation by activation of a non-activated methylene group (46:46′=1:1). An electron-donating methoxy group in the phenyl moiety renders the benzylic C−H bond more electron-rich and shifts the ratio towards the 6-membered ring formation (47:47′). This electronic effect is expected for a reaction that proceeds through an electrophilic iron nitrenoid intermediate (see Supporting information for more details).7 Other examples for 5- vs. 6-membered ring formation are shown for 48:48′ and the citronellol derivatives 49:49′. If the formation of a 5-membered ring is completely blocked, the 6-membered cyclic urea can form efficiently as the main product as shown for compound 29.
Finally, we tested this method for late-stage functionalization by converting primary amines into their N-benzoyloxyurea in a single step, followed by iron-catalyzed C−H amination. The resulting C−H amination products 50–53 (52–95 % yield) are shown in Figure 2 d, which were obtained in overall two steps from 2-aminoindane, N-tosyl tryptamine, and n-dodecylamine. Interestingly, the methyl ester of phenylalanine was converted into the cyclic urea 53 with high diastereoselectivity (>20:1 dr).
It is also noteworthy, that the Fe/phen-catalyzed C−H amination is easily scalable. For example, through C(sp3)-H activation of a non-activated C−H bond, imidazolidine-2-one 42 was obtained in 94 % yield on a 1 mmol scale.
Our new method is clearly distinguished from reported iron catalyzed C−H aminations which most frequently employ organic azides as substrates or reagents, require hypervalent iodine oxidants, or need elaborate ligands for iron coordination, or a combination thereof.13, 14 For example, with respect to azide substrates, Betley14f and Che14o recently reported ring-closing C(sp3)-H aminations of organic azides to pyrrolidines using an iron-dipyrrinato complex and iron porphyrin complex with axially coordinated N-heterocyclic carbene ligands, respectively. De Bruin and van der Vlugt used an iron catalyst with a redox-active pyridine-aminophenol ligand for the same conversion.14n Plietker reported an intramolecular C(sp3)-H amination of alkylaryl azides using a nucleophilic iron carbonyl nitroso complex.14m Beyond azides, sulfamate esters have been used as substrates for intramolecular C(sp3)-H aminations. For example, White14e reported an iron-phthalocyanine catalyzed intramolecular C−H amination of sulfonate esters and Che14g used an interesting quinquepyridine iron catalyst. But both studies required PhI(OAc)2 as oxidant. In contrast, N-benzoyloxyurea have not been reported for iron-catalyzed C−H aminations. They are easily synthesized from primary and secondary amines in two steps and, as demonstrated in this study, undergo C−H aminations under mild “open-flask” reaction conditions using iron dichloride together with 1,10-phenanthroline as a simple and readily available catalyst. While most reported C(sp3)-H amination reactions only provide satisfactory results for aminations of C(sp3)−H bonds activated by arenes, alkenes, alkynes, ether, or amines, our method is applicable to both activated and non-activated C−H bonds.
A proposed mechanism is shown in Figure 3. The active catalyst, iron(II) bound to one or two phen ligands,15 coordinates to the N-benzoyloxyurea and upon base-induced deprotonation of the N-H group and subsequent release of the benzoate leaving group, an intermediate iron nitrenoid (I) is formed. This iron nitrenoid in its triplet state undergoes a 1,5-hydrogen atom transfer (HAT) to generate the radical intermediate II, followed by a rapid radical rebound to establish a new C−N bond (III). Release of the product then regenerates the iron catalyst for a new catalytic cycle. A number of control experiments support this mechanism (see Supporting information for more details). Using FeCl3⋅6 H2O or Fe(OTf)3 as the catalyst instead of FeCl2⋅4 H2O provided significantly reduced yields of 40 % and 46 %, respectively (Table 1, entries 11 and 12), reinforcing that the active catalyst under standard conditions contains Fe in the oxidation state +2. Allylic C−H amination of the substrate (Z)-1 b provided a product mixture in which the majority of the double bond is isomerized to the E-alkene (E)-24 (Figure 3 a). This experiment supports a radical mechanism through an intermediate allylic radical. A stepwise hydrogen abstraction followed by radical rebound instead of a concerted nitrene insertion is also supported by an experiment that revealed loss of stereochemical information in the course of the C−H amination of (S)-1 c to rac-30 (Figure 3 b). Finally, determination of relative initial rates for the substrate 1 a and its deuterated analogue 1 a′ provided a kinetic isotope effect of 1.2, revealing that the C−H cleavage is not the rate determining step of the overall C−H bond amination process but probably rather the N-O cleavage leading to the nitrenoid intermediate (Figure 3 c). An intramolecular competition experiment with substrate 1 d provided a significantly higher KIE of 2.2 as determined from the product ratio 6′:6′′, which is consistent with a different rate for homolytic C−H vs. C-D bond cleavage in a stepwise radical mechanism.
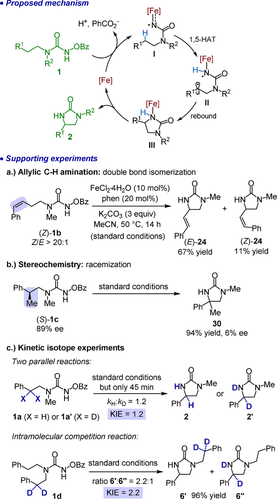
Proposed mechanism and supporting experiments. HAT=hydrogen atom transfer.
In conclusion, we here reported that iron dichloride in combination with 1,10-phenanthroline catalyzes the ring-closing C−H amination of N-benzoyloxyurea to smoothly form imidazolidin-2-ones in high yields. The new method combines several attractive features: First, the catalyst is very simple and readily available without the need for an elaborate organic ligand that needs to be synthesized in a multistep fashion. Second, the reaction conditions are mild and not sensitive to moisture or air (open flask conditions). Third, the C−H amination reaction is surprisingly general and applicable to benzylic, allylic, propargylic, and completely non-activated aliphatic C(sp3)−H bonds, and even works for C(sp2)−H bonds. Finally, the formed imidazolidin-2-ones are important motifs in bioactive compounds and precursors of valuable vicinal diamines.16 It is likely that this new method will become widely applied as a new tool in organic synthesis.
Acknowledgements
L.J. is grateful for a postdoctoral fellowship from the Alexander von Humboldt Foundation. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




