Modification of Fluorescent Photoinduced Electron Transfer (PET) Sensors/Switches To Produce Molecular Photo-Ionic Triode Action†
We thank the Department of Employment and Learning, Northern Ireland, the Engineering and Physical Sciences Research Council (UK), and IAESTE for support.
Abstract
The fluorophore-spacer1-receptor1-spacer2-receptor2 system (where receptor2 alone is photoredox-inactive) shows ionically tunable proton-induced fluorescence off-on switching, which is reminiscent of thermionic triode behavior. This also represents a new extension to modular switch systems based on photoinduced electron transfer (PET) towards the emulation of analogue electronic devices.
Fluorescent photoinduced electron transfer (PET) sensors/switches1–4 are a well-established application of molecular devices, to the point of real-life deployment worldwide in blood electrolyte diagnostics.5–8 Important picosecond laser studies on fluorescent PET sensors/switches have demonstrated the transient existence of radical ion species,9–11 and thus designers can proceed with confidence. As a result of their modular fluorophore-spacer-receptor construction, fluorescent PET systems are very amenable to modification in terms of the format, as well as in terms of the detailed functionalities. The latter approach has yielded many individual examples of sensors and switches based on fluorescence which target important analytes.12–15 On the other hand, the former approach has the potential to set up new areas of endeavor and application, which is exploited here.
The controllable quenching of molecular fluorescence1, 2 can be exploited to build switchable systems which emulate familiar electronic devices. Some of these molecular systems have unique applications which are inconvenient for their electronic counterparts, such as wireless operation in micrometric spaces.16 The first molecular logic gate17–23 1 (an advanced molecular switch24) was a fluorophore-spacer1-receptor1-spacer2-receptor2 system,25, 26 where two photoinduced electron transfer (PET)4, 27 channels arising from the two receptors were controlled by binding H+ and Na+ ions, respectively, and thus the fluorescence output corresponded to photo-ionic AND logic. Strong fluorescence emerges only when all PET processes are suppressed (Figure 1 a).4 Related, but distinct, fluorophore-spacer1-receptor1-spacer2-receptor2 systems,28 where both receptors respond to H+ ions, for example, 2, give rise to fluorescent off-on-off action, which can correspond to ternary logic behavior.22 We now demonstrate aspects of molecular photo-ionic triode action for the first time by structurally mutating the fluorophore-spacer1-receptor1-spacer2-receptor2 system 1 into a novel format exemplified by 3, where the convenient photoredox capability of receptor2 is removed from 1 (Figure 1 b). Nearly 20 distinct formats of luminescent PET switching systems, each possessing its own defining features and applications, are known.26 Fluorescence off-on switching is, therefore, controlled within 3 by selective ion binding of receptor1. This switching profile is influenced by the orthogonally selective ion binding of receptor2, which is forced into a secondary role (Figure 1 b). The amine receptor1 within 3 would bind H+ instead of alkali and alkaline earth cations. The crown ether receptor2 within 3 would bind alkali and alkaline earth cations instead of H+. This photo-ionic triode action complements molecular all-photonic triode behavior, which was reported recently by Gust, Moore, Moore, and co-workers.29 Molecular all-electronic transistor action, and logic gates arising therefrom, is also known.30, 31 It is also important to note a different conceptual approach to a molecular triode based on PET, as described by Verhoeven and co-workers.32
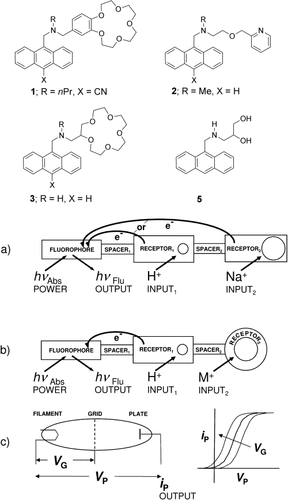
a) A fluorophore for photon transactions and two receptors for ion binding are the three crucial components of the molecular AND logic gate, where the two spacers serve as connectors. b) In a similar vein, the three crucial components of the molecular photo-ionic triode consist of a fluorophore and a principal receptor1 alongside an auxiliary receptor2. The latter endows the system with a way of tuning the input/output (I/O) characteristic curve. c) The three crucial components of the vacuum thermionic triode consist of a filament, plate, and an interspersed grid. This set-up also produces a tunable I/O characteristic.
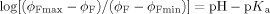 (1)
(1)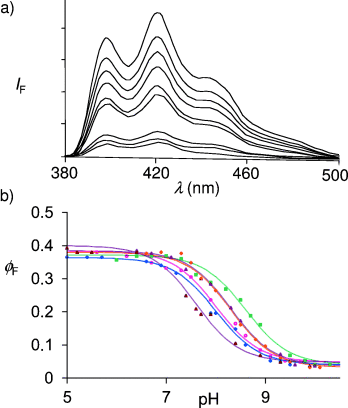
a) Fluorescence emission spectra for 10−5 M 3 in methanol/water (1:1, v/v) with 10−4 M morpholinopropylsulfonic acid in the presence of 0.3 M Me4NCl, when excited at 369 nm. pH adjustments were performed with Me4NOH and HCl. The pH values in order of decreasing fluorescence intensity are: 6.4, 7.6, 8.2, 8.5, 8.7, 8.9, 9.5, 9.8, and 10.3. It is notable that all the spectral features except the quantum yield are essentially independent of the pH value, as expected for fluorescent PET sensors containing fluorophores with ππ* excited states.34 Similar spectra are found when Me4NCl is replaced by other salts (see below). b) Fluorescence quantum yield (ϕF)-pH profiles for 3 in the presence of various chloride salts. The concentrations of monovalent cation salts and divalent cation salts were chosen to minimize ionic strength changes. The salt concentrations were chosen to allow for as much as possible of 3 to be bound to the cation through the crown ether, while respecting solubility limits. Such a choice is enabled by data tables of cation/crown ether binding constants.46 Studies at lower salt concentrations were not conducted since those would require dissection of the ϕF-pH profiles into metal-free and metal-bound components, with large attendant uncertainties. The cations employed are: 0.3 M Me4N+ (filled squares), 0.3 M Na+ (open diamonds), 0.3 M K+ (filled triangles), 0.1 M Ca2+ (filled diamonds), 0.1 M Sr2+ (open circles), and 0.1 M Ba2+ (open triangles). The full lines are calculated according to Equation (1), by employing the experimentally determined parameters pKa, ϕFmax, and ϕFmin from Table 1.
The pKa values determined by fluorescence spectroscopy agree with the corresponding values obtained by absorption spectroscopy, even though the latter values are only estimates because of the small absorption changes that are seen (Table 1). This is as expected for fluorescent PET sensors and switches carrying fluorophores with ππ* excited states,25, 34 since only ϕF is pH-dependent and since the pKa value of the excited state is essentially identical to that of the ground state in these cases. The latter feature contributes to the operational simplicity of fluorescent PET systems compared with other sensing approaches based on fluorescence.4 The electrostatic repulsion between the cation bound by the crown ether and the protonated amine controls the shift of the pKa value (Table 1). Me4N+ was employed to establish the control situation where the crown ether receptor2 is left unbound. Sizeable ΔpKa values of 0.3, 0.3, 0.6, 0.6, and 1.0 are found with K+, Ca2+, Na+, Sr2+, and Ba2+, respectively. As a consequence of the good geometric fit of an Na+ ion into the [15]crown-5 ether cavity,36 it produces a substantial effect, despite its single charge. A lariat action36 from the amine side chain probably contributes to the larger dications being so effective. The tunability of the present system is already significant and sufficient for triode action, similar to the electronic version (shown schematically in Figure 1 c, right panel). Nevertheless, it should now be possible to apply the concept to other receptor pairs so that even larger tunabilities can be reached. An important issue in the development of real-life applications7 is also addressed here—the variation of the pH value around a “normal” value (pHnormal) is most sensitively registered by a molecular sensor if its pKa value is matched to the pHnormal value. The tunability of the pKa value of compound 3 offers a way to make this match.
|
Cation |
pKa 3 |
pKa 3[b] |
ϕFmax 3 |
ϕFmin 3 |
pKa 5 |
pKa 5[b] |
ϕFmax 5 |
ϕFmin 5 |
|---|---|---|---|---|---|---|---|---|
|
Me4N+ |
8.6 |
8.3 |
0.37 |
0.040 |
8.2 |
8.2 |
0.37 |
0.024 |
|
Na+ |
8.0 |
8.0 |
0.36 |
0.040 |
8.2 |
7.9 |
0.38 |
0.034 |
|
K+ |
8.3 |
8.0 |
0.38 |
0.036 |
8.3 |
8.5 |
0.36 |
0.028 |
|
Ca2+ |
8.3 |
8.3 |
0.38 |
0.032 |
8.0 |
–[c] |
0.37 |
0.026 |
|
Sr2+ |
8.0 |
–[c] |
0.38 |
0.048 |
8.0 |
–[c] |
0.36 |
0.036 |
|
Ba2+ |
7.6 |
–[c] |
0.40 |
0.048 |
8.3 |
–[c] |
0.38 |
0.035 |
- [a] Conditions as given in Figure 2. The fluorescence-based pKa values, which were determined according to Equation (1), have uncertainties of ±0.1. The ϕF values have uncertainties of ±10 % and were determined by comparison with secondary standards in Ref. 35. [b] Data estimated by analysis of small H+-induced changes in the UV absorption spectra according to the corresponding version of Equation (1). [c] Spectral changes are too small to permit an estimate to be made.
We have here a rarely noted supramolecular substituent effect37 on pKa values, where the cation serves as the substituent. Physical organic chemistry usually deals with the effects of substituents which are covalently attached to the structure carrying the reactive site.38 Although many cases of ion-induced pKa shifts are available,39–43 the concept of photo-ionic triode action is unprecedented. An elegantly tunable fluorescent PET sensor for glucose developed by James and Shinkai44 does not correspond to photo-ionic triode action. The essential contribution of the [15]crown-5 ether module to the triode action of 3 is demonstrated by the finding that the pKa values of control compound 5 (which is devoid of a crown ether unit) are essentially independent of the cation at 8.15±0.15 (Table 1).
To conclude, the tunable I/O characteristic of a molecular photo-ionic device which emulates thermionic triode behavior has been demonstrated for the first time by implementing a new format of fluorescent PET switches. The three-electrode philosophy of the triode is also followed in the photo-ionic system by the use of three active units within structure 3 (Figure 1 b,c). Another key aspect of triode behavior, that is, signal amplification, is well-known in other chemical systems.45
Dedicated to Prof. Seiji Shinkai




