Gated Electron Sharing Within Dynamic Naphthalene Diimide-Based Oligorotaxanes†
Alyssa-Jennifer Avestro
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDaniel M. Gardner
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Nicolaas A. Vermeulen
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorEleanor A. Wilson
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Severin T. Schneebeli
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Adam C. Whalley
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Matthew E. Belowich
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorProf. Raanan Carmieli
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorCorresponding Author
Prof. Michael R. Wasielewski
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewskihttp://stoddart.northwestern.eduSearch for more papers by this authorCorresponding Author
Prof. J. Fraser Stoddart
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewskihttp://stoddart.northwestern.eduSearch for more papers by this authorAlyssa-Jennifer Avestro
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDaniel M. Gardner
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Nicolaas A. Vermeulen
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorEleanor A. Wilson
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Severin T. Schneebeli
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Adam C. Whalley
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorDr. Matthew E. Belowich
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorProf. Raanan Carmieli
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Search for more papers by this authorCorresponding Author
Prof. Michael R. Wasielewski
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewskihttp://stoddart.northwestern.eduSearch for more papers by this authorCorresponding Author
Prof. J. Fraser Stoddart
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewski http://stoddart.northwestern.edu
Center for the Chemistry of Integrated Systems (CCIS) and Argonne-Northwestern Solar Energy Research (ANSER) Center, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA) http://chemgroups.northwestern.edu/wasielewskihttp://stoddart.northwestern.eduSearch for more papers by this authorWe would like to thank Dr. Saman Shafaie for collecting high-resolution mass spectrometric data, Drs. Charlotte Stern and Amy A. Sargeant for single-crystal structure determination, and Drs. James M. Holcroft and Marco Frasconi for helpful discussions. This research is part (Project 34-947) of the Joint Center of Excellence in Integrated Nano-Systems (JCIN) at King Abdul-Aziz City for Science and Technology (KACST) and Northwestern University (NU). We would like to thank both KACST and NU for their continued support of this research. M.R.W. is supported by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy, DOE, under grant no. DE-FG02-99ER-14999. A.-J.A. gratefully acknowledges the National Science Foundation (NSF) for the award of a Graduate Research Fellowship (GRF) under Grant No. DGE-0824162. D.M.G. was supported by the Department of Defense through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program. S.T.S. thanks the International Institute for Nanotechnology (IIN) at NU for an IIN Postdoctoral Fellowship and the QUEST high-performance computing center at NU for a research allocation of computer time. R.C. is supported by the ANSER Center, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001059.
Abstract
The controlled self-assembly of well-defined and spatially ordered π-systems has attracted considerable interest because of their potential applications in organic electronics. An important contemporary pursuit relates to the investigation of charge transport across noncovalently coupled components in a stepwise fashion. Dynamic oligorotaxanes, prepared by template-directed methods, provide a scaffold for directing the construction of monodisperse one-dimensional assemblies in which the functional units communicate electronically through-space by way of π-orbital interactions. Reported herein is a series of oligorotaxanes containing one, two, three and four naphthalene diimide (NDI) redox-active units, which have been shown by cyclic voltammetry, and by EPR and ENDOR spectroscopies, to share electrons across the NDI stacks. Thermally driven motions between the neighboring NDI units in the oligorotaxanes influence the passage of electrons through the NDI stacks in a manner reminiscent of the conformationally gated charge transfer observed in DNA.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201309680_sm_miscellaneous_information.pdf6.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. Gust, T. A. Moore, A. L. Moore, Acc. Chem. Res. 1993, 26, 198–205;
- 1bC. J. Murphy, M. R. Arkin, Y. Jenkins, N. D. Ghatlia, S. H. Bossmann, N. J. Turro, J. K. Barton, Science 1993, 262, 1025–1029;
- 1cJ. C. Genereux, J. K. Barton, Chem. Rev. 2010, 110, 1642–1662.
- 2
- 2aM. R. Wasielewski, Acc. Chem. Res. 2009, 42, 1910–1921;
- 2bR. Bhosale, J. Mišek, N. Sakai, S. Matile, Chem. Soc. Rev. 2010, 39, 138–149;
- 2cF. Garo, R. Häner, Angew. Chem. 2012, 124, 940–943;
10.1002/ange.201103295 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 916–919;
- 2dP. Frischmann, K. Mahata, F. Würthner, Chem. Soc. Rev. 2013, 42, 1847–1870;
- 2eG. Sforazzini, E. Orentas, A. Bolag, N. Sakai, S. Matile, J. Am. Chem. Soc. 2013, 135, 12082–12090.
- 3
- 3aA. Takai, T. Yasuda, T. Ishizuka, T. Kojima, M. Takeuchi, Angew. Chem. 2013, 125, 9337–9341;
10.1002/ange.201302587 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9167–9171;
- 3bA. C. Fahrenbach, S. C. Warren, J. T. Incorvati, A.-J. Avestro, J. C. Barnes, J. F. Stoddart, B. A. Grzybowski, Adv. Mater. 2013, 25, 331–348.
- 4
- 4aV. Percec, M. Glodde, T. K. Bera, Y. Miura, I. Shiyanovskaya, K. D. Singer, V. S. K. Balagurusamy, P. A. Heiney, I. Schnell, A. Rapp, H.-W. Spiess, S. D. Hudson, H. Duan, Nature 2002, 417, 384–387;
- 4bT. Aida, E. W. Meijer, S. I. Stupp, Science 2012, 335, 813–817;
- 4cH. Usta, M. D. Yilmaz, A.-J. Avestro, D. Boudinet, M. Denti, J. F. Stoddart, A. Facchetti, Adv. Mater. 2013, 25, 4327–4334.
- 5
- 5aW. B. Davis, W. A. Svec, M. A. Ratner, M. R. Wasielewski, Nature 1998, 396, 60–63;
- 5bC. A. Hunter, S. Tomas, J. Am. Chem. Soc. 2006, 128, 8975–8979;
- 5cP. M. Beaujuge, J. M. J. Fréchet, J. Am. Chem. Soc. 2011, 133, 20009–20029;
- 5dA. Coskun, J. M. Spruell, G. Barin, W. R. Dichtel, A. H. Flood, Y. Y. Botros, J. F. Stoddart, Chem. Soc. Rev. 2012, 41, 4827–4859.
- 6
- 6aH. Oevering, M. N. Paddon-Row, M. Heppener, A. M. Oliver, E. Cotsaris, J. W. Verhoeven, N. S. Hush, J. Am. Chem. Soc. 1987, 109, 3258–3269;
- 6bE. A. Weiss, M. J. Ahrens, L. E. Sinks, A. V. Gusev, M. A. Ratner, M. R. Wasielewski, J. Am. Chem. Soc. 2004, 126, 5577–5584;
- 6cM. Scott, T. Miura, A. Butler Ricks, Z. E. X. Dance, E. M. Giacobbe, M. T. Colvin, M. R. Wasielewski, J. Am. Chem. Soc. 2009, 131, 17655–17666.
- 7
- 7aF.-R. F. Fan, J. Yang, L. Cai, D. W. Price, Jr., S. M. Dirk, D. V. Kosynkin, Y. Yao, A. M. Rawlett, J. M. Tour, A. J. Bard, J. Am. Chem. Soc. 2002, 124, 5550–5560;
- 7bS. A. Serron, S. A. Aldridge III, C. N. Fleming, R. M. Danell, M.-H. Bailk, M. Sykora, D. M. Dettelbaum, T. J. Meyer, J. Am. Chem. Soc. 2004, 126, 14506–14514;
- 7cT. M. Wilson, T. A. Zeidan, M. Hariharan, F. D. Lewis, M. R. Wasielewski, Angew. Chem. 2010, 122, 2435–2438;
10.1002/ange.200907339 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2385–2388;
- 7dS. Sengupta, D. Ebeling, S. Patwardhan, X. Zhang, H. von Berlepsch, C. Böttcher, V. Stepanenko, S. Uemura, C. Hentschel, H. Fuchs, F. C. Grozema, L. D. A. Siebbeles, A. R. Holzwarth, L. Chi, F. Würthner, Angew. Chem. 2012, 124, 6484–6488;
10.1002/ange.201201961 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6378–6382.
- 8
- 8aY. Morisaki, S. Ueno, A. Saeki, A. Asano, S. Seki, Y. Chujo, Chem. Eur. J. 2012, 18, 4216–4224;
- 8bM. Wielopolski, A. Molina-Ontoria, C. Schubert, J. T. Margraf, E. Krokos, J. Kirschner, A. Gouloumis, T. Clark, D. M. Guldi, N. Martin, J. Am. Chem. Soc. 2013, 135, 10372–10381.
- 9J. E. Bullock, R. Carmieli, S. M. Mickley, J. Vura-Weis, M. R. Wasielewski, J. Am. Chem. Soc. 2009, 131, 11919–11929.
- 10
- 10aS. Anderson, H. L. Anderson, J. K. M. Sanders, Acc. Chem. Res. 1993, 26, 469–475;
- 10bR. Cacciapaglia, L. Manodolini, Chem. Soc. Rev. 1993, 22, 221–231;
- 10cR. Hoss, F. Vögtle, Angew. Chem. 1994, 106, 389–398; Angew. Chem. Int. Ed. Engl. 1994, 33, 375–384;
- 10dG. A. Breault, C. A. Hunter, P. C. Mayers, Tetrahedron 1999, 55, 5265–5293;
- 10eT. J. Hubin, D. H. Busch, Coord. Chem. Rev. 2000, 200, 5–52;
- 10fM.-J. Blanco, J.-C. Chambron, M. C. Jiménez, J.-P. Sauvage, Top. Stereochem. 2002, 23, 125–173;
- 10gD. H. Busch, Top. Curr. Chem. 2005, 249, 1–65;
- 10hC. D. Meyer, C. S. Joiner, J. F. Stoddart, Chem. Soc. Rev. 2007, 36, 1705–1723.
- 11
- 11aT. R. Kelly, R. L. Xie, C. K. Weinreb, T. Bregant, Tetrahedron Lett. 1998, 39, 3675–3678;
- 11bY. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim, H. Yan, Adv. Mater. 2003, 15, 353–389;
- 11cL. Palmer, S. I. Stupp, Acc. Chem. Res. 2008, 41, 1674–1684.
- 12Y.-L. Wu, K. E. Brown, M. R. Wasielewski, J. Am. Chem. Soc. 2013, 135, 13322–13325.
- 13
- 13aN. Sakai, M. Lista, O. Kel, S. Sakurai, D. Emery, J. Mareda, E. Vauthey, S. Matile, J. Am. Chem. Soc. 2011, 133, 15224–15227;
- 13bM. Lista, J. Areephong, N. Sakai, S. Matile, J. Am. Chem. Soc. 2011, 133, 15228–15230;
- 13cN. Sakai, S. Matile, J. Am. Chem. Soc. 2011, 133, 18542–18545.
- 14aY. Yamauchi, M. Yoshizawa, M. Akita, M. Fujita, J. Am. Chem. Soc. 2010, 132, 960–966;
- 14bM. Kiguchi, T. Takahashi, Y. Takahashi, Y. Yamauchi, T. Murase, M. Fujita, T. Tada, S. Watanabe, Angew. Chem. 2011, 123, 5826–5829;
10.1002/ange.201100431 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5708–5711.
- 15M. Kiguchi, O. Tal, S. Wohlthat, F. Pauly, M. Krieger, D. Djukic, J. C. Cueves, J. M. van Ruitenbeek, Phys. Rev. Lett. 2008, 101, 046801.
- 16
- 16aP. T. Glink, A. I. Oliva, J. F. Stoddart, A. J. P. White, D. J. Williams, Angew. Chem. 2001, 113, 1922–1927;
10.1002/1521-3757(20010518)113:10<1922::AID-ANGE1922>3.0.CO;2-T Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1870–1875;10.1002/1521-3773(20010518)40:10<1870::AID-ANIE1870>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 16bM. Horn, J. Ihringer, P. T. Glink, J. F. Stoddart, Chem. Eur. J. 2003, 9, 4046–4054.
- 17
- 17aS. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. 2002, 114, 938–993;
10.1002/1521-3757(20020315)114:6<938::AID-ANGE938>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2002, 41, 898–952;10.1002/1521-3773(20020315)41:6<898::AID-ANIE898>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 17bJ. M. Lehn, Chem. Soc. Rev. 2007, 36, 151–160;
- 17cM. E. Belowich, J. F. Stoddart, Chem. Soc. Rev. 2012, 41, 2003–2024;
- 17dY. Jin, C. Yu, R. J. Denman, W. Zhang, Chem. Soc. Rev. 2013, 42, 6634–6654.
- 18
- 18aM. E. Belowich, C. Valente, R. A. Smaldone, D. C. Friedman, J. Thiel, L. Cronin, J. F. Stoddart, J. Am. Chem. Soc. 2012, 134, 5243–5261;
- 18bA.-J. Avestro, M. E. Belowich, J. F. Stoddart, Chem. Soc. Rev. 2012, 41, 5869–6216.
- 19
- 19aS. C. Bhosale, S. V. Bhosale, S. K. Bhargava, Org. Biomol. Chem. 2012, 10, 6455–6468;
- 19bZ. Zhu, C. J. Cardin, Y. Gan, H. M. Colquhoun, Nat. Chem. 2010, 2, 653–660;
- 19cA. Das, S. Ghosh, Macromolecules 2013, 46, 3939–3949;
- 19dM. B. Avinash, T. Govindaraju, Adv. Mater. 2012, 24, 3905–3922;
- 19eM. B. Avinash, E. Verheggen, C. Schmuck, T. Govindaraju, Angew. Chem. 2012, 124, 10470–10474;
10.1002/ange.201204608 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10324–10328;
- 19fR. S. Lokey, Y. Kwok, V. Guelev, C. J. Pursell, L. H. Hurley, B. L. Iverson, J. Am. Chem. Soc. 1997, 119, 7202–7210;
- 19gA. R. Smith, B. L. Iverson, J. Am. Chem. Soc. 2013, 1035, 12783–12789;
- 19hS. V. Bhosale, C. H. Jani, S. J. Langford, Chem. Soc. Rev. 2008, 37, 331–342;
- 19iN. Sakai, J. Mareda, E. Vauthey, S. Matile, Chem. Commun. 2010, 46, 4225–4237;
- 19jS. Tu, S. H. Kim, J. Joseph, D. A. Modarelli, J. R. Parquette, ChemPhysChem 2013, 14, 1609–1617;
- 19kH. Shao, T. Nguyen, N. C. Romano, D. A. Modarelli, J. R. Parquette, J. Am. Chem. Soc. 2009, 131, 16374–16376;
- 19lS. Guha, F. S. Goodson, S. Roy, L. J. Corson, C. A. Gravenmier, S. Saha, J. Am. Chem. Soc. 2011, 133, 15256–15259;
- 19mN. Ponnuswamy, F. B. L. Cougnon, J. M. Clough, G. D. Pantoş, J. K. M. Sanders, Science 2012, 338, 783–785;
- 19nS. P. Black, A. R. Stefankiewicz, M. M. J. Smulders, D. Sattler, C. A. Schalley, J. R. Nitschke, J. K. M. Sanders, Angew. Chem. 2013, 125, 5861–5864;
10.1002/ange.201209708 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 5749–5752;
- 19oC. Shao, M. Stolte, F. Würthner, Angew. Chem. 2013, 125, 7630–7634; Angew. Chem. Int. Ed. 2013, 52, 7482–7486;
- 19pS. Sengupta, F. Würthner, Acc. Chem. Res. 2013, 46, 2498–2512;
- 19qY. S. Chong, W. R. Carroll, W. G. Burns, M. D. Smith, K. D. Shimizu, Chem. Eur. J. 2009, 15, 9117–9126;
- 19rX. Guo, D. Zhang, D. Zhu, Adv. Mater. 2004, 16, 125–130.
- 20X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski, S. R. Marder, Adv. Mater. 2011, 23, 268–284.
- 21The peak integrations are consistent with the ratios of the constitutionally heterotopic outer and inner rings (blue and red in the 1H NMR spectra) present in the [n]NDIxR series—that is, 2:1 and 1:1 for x=3 and 4, respectively.
- 22All efforts resulted in single crystals that were either twinned or unable to diffract with high enough resolution. The ability of the rings to rotate or twist around the dumbbells in order to accommodate the sterically bulky groups could result conceivably in multiple favorable co-conformations in the solid state. Surprisingly, out of the entire [n]NDIxR series, the most successful crystallizations were achieved from solutions (0.9 mg mL−1) of [5]NDI4R in CH2Cl2/n-heptane grown at 0 °C, yet these crystals were only able to diffract up to 2.8 Å using a Cu beam source. We attribute this observation to the more rigid structure afforded by multiple interactions when x=4.
- 23Crystal data for the [3]rotaxane [3]Ph2R: C95H109F12N9O14P2, MW=1890.891, monoclinic, space group P1n1, a=24.5464(18), b=15.2507(10), c=25.4313(19) Å, β=95.403(5)°, V=9477.9(12) Å3, T=100(2) K, Z=4, ρcalc=1.325 g cm−3, μ(Cu-Kα)=1.188, F(000)=3968. Independent measured reflections 16 367. R1=0.1117, wR2=0.3415 for 9167 independent observed reflections [2θ≤57.62°, I>2σ(I)]. CCDC 963939 ([3]Ph2R) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 24The computed structure of [3]NDI2R was obtained by modeling the NDI units directly onto the X-ray crystal structure obtained for the related [3]rotaxane [3]Ph2R (see Ref. [23]), which is devoid of the NDI functionality, and performing an unconstrained geometry optimization at the B3LYP-D3/6-31G** level of theory (Ref. [25]). Density functional theory (DFT) measurements were only carried out on the [2]rotaxane [3]NDI2R since calculations on the higher order [n]rotaxanes failed to converge in a reasonable amount of time.
- 25
- 25aC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789;
- 25bA. D. Becke, J. Chem. Phys. 1993, 98, 1372–1377;
- 25cS. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 26UV/Vis spectroscopy (Figure S27) of the [n]NDIxR series (5.0 μM in CH2Cl2) reveals the existence of three absorption bands between 330 and 400 nm characteristic of other NDI derivatives in the literature (Ref. [19]). An intensity reversal of the absorptions centered at 360 and 380 nm, relative to one another as the number of NDI units is increased, may indicate weak exciton coupling between the NDI units in the [n]rotaxanes in CH2Cl2 solution. For more details, see:
- 26aM. Kasha, H. R. Rawles, M. L. El-Bayoumi, Pure Appl. Chem. 1965, 11, 371–392;
- 26bA. D. Q. Li, W. Wang, L. Q. Wang, Chem. Eur. J. 2003, 9, 4594–4601;
- 26cJ. M. Giaimo, J. V. Lockard, L. E. Sinks, A. M. Scott, T. M. Wilson, M. R. Wasielewski, J. Phys. Chem. A 2008, 112, 2322–2330.
- 27Since subjecting the [n]rotaxane samples (1.0 mM in Ar-purged CH2Cl2) to multiple redox cycles beyond −1.44 V resulted in decomposition of the molecules, presumbly as a consequence of the irreversible reduction of the pyridine ring, applying high negative potentials were avoided when recording CV spectra. For more details, see: A. Cisak, P. J. Elving, Electrochim. Acta 1965, 10, 935–946.
- 28Sharing of an unpaired spin between identical monomeric units is characterized by narrowing of the EPR spectral linewidth by a factor proportional to the square root of the number of monomers involved, as given by Equation (1):
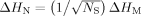 which relates the linewidth of the monomer (ΔHM) to the observed linewidth (ΔHN) when an unpaired spin is shared over NS molecular sites. For more details, see: J. R. Norris, R. A. Uphaus, H. L. Crespi, J. J. Katz, Proc. Natl. Acad. Sci. USA 1971, 68, 625–628.
which relates the linewidth of the monomer (ΔHM) to the observed linewidth (ΔHN) when an unpaired spin is shared over NS molecular sites. For more details, see: J. R. Norris, R. A. Uphaus, H. L. Crespi, J. J. Katz, Proc. Natl. Acad. Sci. USA 1971, 68, 625–628.
- 29H. Kurreck, B. Kirste, W. Lubitz, Electron Nuclear Double Resonance Spectroscopy of Radicals in Solution, VCH, New York, 1988.
- 30
- 30aJ.-F. Penneau, B. J. Stallman, P. H. Kasai, L. L. Miller, Chem. Mater. 1991, 3, 791–796;
- 30bL. L. Miller, R. G. Duan, Y. Hong, I. Tabakovic, Chem. Mater. 1995, 7, 1552–1557;
- 30cL. L. Miller, K. R. Mann, Acc. Chem. Res. 1996, 29, 417–423.
- 31
- 31aS. G. Chen, H. M. Branz, S. S. Eaton, P. C. Taylor, R. A. Cormier, B. A. Gregg, J. Phys. Chem. B 2004, 108, 17329–17336;
- 31bY. K. Che, A. Data, X. M. Yang, T. Naddo, J. C. Zhao, L. Zang, J. Am. Chem. Soc. 2007, 129, 6354–6355;
- 31cT. M. Wilson, M. J. Tauber, M. R. Wasielewski, J. Am. Chem. Soc. 2009, 131, 8952–8957.
- 32The term “conformational gating” has been used to describe the thermally driven motion of base pairs to mediate hole transfer within double-stranded DNA. For more examples, see:
- 32aG. I. Likhtenshtein, J. Photochem. Photobiol. A 1996, 96, 79–92;
- 32bS. Delaney, J. K. Barton, J. Org. Chem. 2003, 68, 6475–6483;
- 32cM. A. O’Neill, J. K. Barton, J. Am. Chem. Soc. 2004, 126, 11471–11483;
- 32dE. A. Weiss, M. J. Tauber, R. F. Kelley, M. J. Ahrens, M. A. Ratner, M. R. Wasielewski, J. Am. Chem. Soc. 2005, 127, 11842–11850.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




