Palladium-Catalyzed Asymmetric Allylic Alkylation of 3-Aryloxindoles with Allylidene Dipivalate: A Useful Enol Pivalate Product†
Corresponding Author
Prof. Barry M. Trost
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost===Search for more papers by this authorJames T. Masters
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost
Search for more papers by this authorDr. Aaron C. Burns
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost
Search for more papers by this authorCorresponding Author
Prof. Barry M. Trost
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost===Search for more papers by this authorJames T. Masters
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost
Search for more papers by this authorDr. Aaron C. Burns
Department of Chemistry, Stanford University, Stanford CA 94305-4401 (USA) http://www.stanford.edu/group/bmtrost
Search for more papers by this authorWe thank the National Institutes of Health (R01GM033049) and the National Science Foundation for their generous support of our programs. J.T.M. is supported by an Abbott Laboratories Stanford Graduate Fellowship. We also thank Johnson-Matthey for their generous gift of palladium salts.
Graphical Abstract
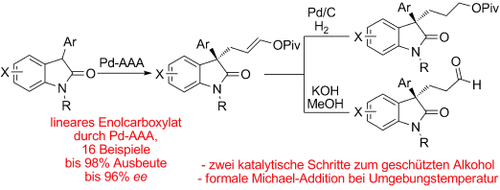
Dreifach A: Die katalytische asymmetrische allylische Alkylierung (AAA) von 3-Aryloxindolen mit Allylidendipivalat wird beschrieben. Die Reaktion liefert stabile, präparativ nützliche Enolpivalate in hohen Ausbeuten und mit exzellenter Regio- und Enantioselektivität. Eine Bandbreite von Substraten wird toleriert, darunter ungeschützte und 3-Heteroaryl-Nucleophile.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209783_sm_miscellaneous_information.pdf3.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aB. M. Trost, D. L. van Vranken, Chem. Rev. 1996, 96, 395;
- 1bB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921;
- 1cB. M. Trost, M. R. Machacek, A. Aponick, Acc. Chem. Res. 2006, 39, 747;
- 1dZ. Lu, S. Ma, Angew. Chem. 2008, 120, 264; Angew. Chem. Int. Ed. 2008, 47, 258;
- 1eB. M. Trost, Org. Process Res. Dev. 2012, 16, 185.
- 2For reviews covering the asymmetric synthesis of quaternary stereocenters, see:
- 2aE. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 402;
10.1002/(SICI)1521-3757(19980216)110:4<402::AID-ANGE402>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 388;10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google Scholar
- 2bJ. Christoffers, A. Mann, Angew. Chem. 2001, 113, 4725;
10.1002/1521-3757(20011217)113:24<4725::AID-ANGE4725>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4591;10.1002/1521-3773(20011217)40:24<4591::AID-ANIE4591>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 2cC. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 5363;
- 2dJ. Christoffers, A. Baro, Adv. Synth. Catal. 2005, 347, 1473;
- 2eB. M. Trost, C. Jiang, Synthesis 2006, 369;
- 2f Quaternary Stereocenters (Eds.: ), Wiley-VCH, Weinheim, 2005;
- 2gJ. P. Das, I. Marek, Chem. Commun. 2011, 47, 4593.
- 3
- 3aB. M. Trost, Science 1991, 254, 1471;
- 3bB. M. Trost, Angew. Chem. 1995, 107, 285;
10.1002/ange.19951070304 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 259.
- 4P. A. Wender, V. A. Verma, T. J. Paxton, T. H. Pillow, Acc. Chem. Res. 2008, 41, 40.
- 5
- 5aB. M. Trost, C. B. Lee, J. M. Weiss, J. Am. Chem. Soc. 1995, 117, 7247;
- 5bB. M. Trost, C. B. Lee, J. Am. Chem. Soc. 1998, 120, 6818;
- 5cB. M. Trost, C. B. Lee, J. Am. Chem. Soc. 2001, 123, 3671; B. M. Trost, C. B. Lee, J. Am. Chem. Soc. 2001, 123, 3687.
- 6See Ref. [5c]. It has been observed that dimethyl methylmalonate reacts with allylidene diacetate to provide the linear product when PPh3 is used as the ligand; however, the use of ligand L1 with this system favors branched material. See also B. M. Trost, J. Vercauteren, Tetrahedron Lett. 1985, 26, 131.
- 7See
- 7aB. M. Trost, R. Radinov, E. M. Grenzer, J. Am. Chem. Soc. 1997, 119, 7879;
- 7bB. M. Trost, X. Ariza, J. Am. Chem. Soc. 1999, 121, 10727;
- 7cB. M. Trost, G. M. Schroeder, Chem. Eur. J. 2005, 11, 174, and references therein.
- 8For reviews covering the biological significance of 3,3-disubstituted oxindoles and their derivatives as well as the asymmetric synthesis of these compounds, see:
- 8aA. B. Dounay, L. E. Overman, Chem. Rev. 2003, 103, 2945;
- 8bC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209;
- 8cC. V. Galliford, K. A. Scheidt, Angew. Chem. 2007, 119, 8902;
10.1002/ange.200701342 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8748;
- 8dA. Steven, L. E. Overman, Angew. Chem. 2007, 119, 5584; Angew. Chem. Int. Ed. 2007, 46, 5488;
- 8eB. M. Trost, M. K. Brennan, Synthesis 2009, 3003;
- 8fF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381;
- 8gJ. E. M. N. Klein, R. J. K. Taylor, Eur. J. Org. Chem. 2011, 6821, and references therein. For the asymmetric allylic alkylation and benzylation of oxindole nucleophiles using Mo- and Pd-AAA, see:
- 8hB. M. Trost, M. U. Frederiksen, Angew. Chem. 2005, 117, 312;
10.1002/ange.200460335 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 308;
- 8iB. M. Trost, Y. Zhang, J. Am. Chem. Soc. 2006, 128, 4590;
- 8jB. M. Trost, Y. Zhang, J. Am. Chem. Soc. 2007, 129, 14548;
- 8kB. M. Trost, Y. Zhang, Chem. Eur. J. 2010, 16, 296;
- 8lB. M. Trost, L. C. Czabaniuk, J. Am. Chem. Soc. 2010, 132, 15534;
- 8mB. M. Trost, J. Xie, J. D. Sieber, J. Am. Chem. Soc. 2011, 133, 20611.
- 9
- 9aG. S. Zweifel, M. H. Nantz, Modern Organic Synthesis, Freeman, New York, 2007;
- 9b Organocatalytic Enantioselective Conjugate Addition Reactions (Eds.: ), RSC Catalysis Series No. 5, RSC Publishing, Oxford, 2010.
- 10R. He, C. Ding, K. Maruoka, Angew. Chem. 2009, 121, 4629; Angew. Chem. Int. Ed. 2009, 48, 4559. Following our submission of this manuscript, a report from Lu and co-workers on chiral phosphine catalyzed asymmetric Michael additions of N-Boc oxindoles was published online ( F. Zhong, X. Dou, X. Han, W. Yao, Q. Zhu, Y. Meng, Y. Lu, Angew. Chem. 2012, DOI: ; Angew. Chem. Int. Ed. 2012, DOI: ). However, this method suffers from the same operational drawbacks mentioned (i.e. high dilution and cryogenic temperatures) and the singular example of an asymmetric addition into acrolein proceeds with more modest (71 %) ee.
- 11For recent progress in the conjugate addition of 3-alkyloxindoles to β-substituted enals, see:
- 11aP. Galzerano, G. Bencivenni, F. Pesciaioli, A. Mazzanti, B. Giannichi, L. Sambri, G. Bartoli, P. Melchiorre, Chem. Eur. J. 2009, 15, 7846;
- 11bN. Bravo, I. Mon, X. Companyó, A.-N. Alba, A. Moyano, R. Rios, Tetrahedron Lett. 2009, 50, 6624.
- 12For a larger-scale (67.5 mmol) synthesis of allylidene dipivalate, see M. Lombardo, S. Licciulli, F. Pasi, G. Angelici, C. Trombini, Adv. Synth. Catal. 2005, 347, 2015.
- 13When electron-deficient nucleophiles were reacted in THF/tBuOH, a decrease in regioselectivity was observed. As a result, these reactions were performed in PhMe/tBuOH (see Supporting Information for full experimental details and solvent selections for each nucleophile).
- 14Attempts to extend this methodology to additional substrates including 3-alkyloxindoles are the subject of continued study.
- 15L. E. Overman, Y. Shin, Org. Lett. 2007, 9, 339.
- 16PhMe was used as the reaction solvent, and products 5 k–5 o were obtained in ca. 92–97 % purity; see Supporting Information for full details.
- 17Further experiments and mechanistic investigations to fully elucidate these interesting results are the subject of continued study.
- 18
- 18aM. Node, X.-J. Hao, K. Fuji, Chem. Lett. 1991, 57;
- 18bW. W. Wilkerson, A. A. Kergaye, S. W. Tam, J. Med. Chem. 1993, 36, 2899;
- 18cM. Node, X.-J. Hao, K. Nishide, K. Fuji, Chem. Pharm. Bull. 1996, 44, 715;
- 18dS. Pellegrino, F. Clerici, A. Contini, S. Leone, T. Pilati, M. L. Gelmi, Tetrahedron 2009, 65, 1995;
- 18eG. Chen, T. D. Cushing, P. Faulder, B. Fisher, X. He, K. Li, Z. Li, W. Liu, L. R. Mcgee, V. Pattaropong, J. L. Seganish, Y. Shin, Z. Wang, “Alkynyl Alcohols as Kinase Inhibitors”, U.S. Patent Application 20110086834, Apr. 14, 2011.
- 19The antipode of aldehyde 9 has previously been synthesized by Maruoka and co-workers (see Scheme 1, Ref. [10], and the Supporting Information). The absolute stereochemistry of enol pivalates 2 and 5 a–5 o is assigned by analogy to 5 k.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.