Intramolecular C(sp3)N Coupling by Oxidation of Benzylic C,N-Dianions†
Jenna L. Jeffrey
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)
Search for more papers by this authorEmily S. Bartlett
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)
Search for more papers by this authorCorresponding Author
Prof. Richmond Sarpong
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)===Search for more papers by this authorJenna L. Jeffrey
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)
Search for more papers by this authorEmily S. Bartlett
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)
Search for more papers by this authorCorresponding Author
Prof. Richmond Sarpong
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720 (USA)===Search for more papers by this authorWe thank Dr. Vishnumaya Bisai (IISER Bhopal), Dr. André Isaacs (College of the Holy Cross (USA)), and Erica D’Amato (visiting undergraduate Amgen Scholar, 2011) for assistance with the preparation of starting materials for an initial study. This work was supported by the NIH-USA (NIGMS RO1 GM86374) and a Research Scholar Grant from the American Cancer Society-USA (RSG-09-017-01-CDD to R.S.). R.S. is a Camille Dreyfus Teacher Scholar. J.L.J. is grateful to the NSF for a graduate fellowship. E.S.B was supported by a Chemical Biology Interface Training Grant from the NIH (T32 GM066698). We are grateful to Eli Lilly, Abbott, and Roche for unrestricted financial support.
Graphical Abstract
Clever verknüpft! Eine intramolekulare C(sp3)-N-Kupplung zur Bildung von Azacyclen wird beschrieben. Die Reaktion verläuft über die Oxidation von benzylischen C,N-Dianionen mit Iod und knüpft an eine frühere Entdeckung bei der Synthese von Lyconadin A an. Die vorliegende Studie nutzt konformativ freie Substrate mit weniger aciden C-H-Bindungen und weniger reaktiven Stickstoffnukleophilen. ZnCl2 wurde als ein wichtiges Additiv identifiziert.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209591_sm_miscellaneous_information.pdf10.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a review on the Hofmann–Löffler–Freytag reaction, see:
- 1aL. Feray, N. Kuznetsov, P. Renaud in Radicals in Organic Synthesis, Vol. 2 (Ed.: ), Wiley-VCH, Weinheim, 2001, pp. 254–256;
- 1bL. Stella in Radicals in Organic Synthesis Vol. 2 (Ed.: ), Wiley-VCH, Weinheim, 2001, pp. 409–426.
- 2For example, the Suárez modification, see: C. G. Francisco, A. J. Herrera, E. Suárez, J. Org. Chem. 2003, 68, 1012–1017.
- 3For an elegant recent C(sp3)N forming reaction involving nitrenium-like ions generated from 1-aza-2-azoniallene salts, see: D. A. Bercovici, M. Brewer, J. Am. Chem. Soc. 2012, 134, 9890–9893.
- 4Several transition-metal-catalyzed oxidative C(sp3)N bond-forming reactions have been reported. However, in all these cases, a primary amine derivative that is attached to an electron-withdrawing group is required. For selected recent accounts, see:
- 4aJ. Du Bois, Org. Process Res. Dev. 2011, 15, 758–762;
- 4bC. G. Espino, J. Du Bois in Modern Rhodium-Catalyzed Organic Reactions (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 379–416.
- 5For PdII-catalyzed oxidative C(sp2)N bond-forming reactions, see:
- 5aT.-S. Mei, X. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 10806–10807; for PdII-catalyzed allylic CH amination, see:
- 5bS. A. Reed, M. C. White, J. Am. Chem. Soc. 2008, 130, 3316–3318. In these cases, amines attached to electron-withdrawing groups are required.
- 6For a review, see: C. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215–1292.
- 7For recent examples of applications in complex molecule synthesis, see:
- 7aP. S. Baran, B. D. Hafensteiner, N. B. Ambhaikar, C. A. Guerrero, J. D. Gallagher, J. Am. Chem. Soc. 2006, 128, 8678–8693;
- 7bC. L. Martin, L. E. Overman, J. M. Rohde, J. Am. Chem. Soc. 2010, 132, 4894–4906;
- 7cW. Zi, W. Xie, D. Ma, J. Am. Chem. Soc. 2012, 134, 9126–9129;
- 7dZ. Zuo, W. Xie, D. Ma, J. Am. Chem. Soc. 2010, 132, 13226–13228.
- 8
- 8aA. Bisai, S. P. West, R. Sarpong, J. Am. Chem. Soc. 2008, 130, 7222–7223;
- 8bS. P. West, A. Bisai, A. D. Lim, R. R. Narayan, R. Sarpong, J. Am. Chem. Soc. 2009, 131, 11187–11194.
- 9R. R. Fraser, T. S. Mansour, S. Savard, J. Org. Chem. 1985, 50, 3232–3234.
- 10F. G. Bordwell, Acc. Chem. Res. 1988, 21, 456–463.
- 11This approximate pKa value is inferred from the reported pKa for toluene, see:
- 11aA. Streitwieser, M. R. Granger, F. Mares, R. A. Wolf, J. Am. Chem. Soc. 1973, 95, 4257–4261;
- 11bA. Streitwieser, D. W. Boerth, J. Am. Chem. Soc. 1978, 100, 755–759.
- 12We anticipated that 4 would undergo lateral lithiation on the basis of the studies of Smith et al., who have previously shown that N′-(2-methylbenzyl)-N,N-dimethylurea and N-(2-methylbenzyl) pivalamide undergo lateral lithiation to form the corresponding dilithiated species. See: K. Smith, G. A. El-Hiti, A. S. Hegazy, Synthesis 2010, 1371–1380.
- 13P. Beak, A. I. Meyers, Acc. Chem. Res. 1986, 19, 356–363.
- 14Coordinated solvents removed for clarity. For a discussion of the aggregation state of the dianion generated upon deprotonation of 1 in THF, see: J. M. Gruver, S. P. West, D. B. Collum, R. Sarpong, J. Am. Chem. Soc. 2010, 132, 13212–13213.
- 15For the influence of additives on alkyllithium basicity, see: For LiX
- 15aD. Seebach, V. W. Bauer, Helv. Chim. Acta 1984, 67, 1972–1988; For amine bases
- 15bT. L. Brown, Pure Appl. Chem. 1970, 23, 447–462;
- 15cB. J. Wakefield, The Chemistry of Organolithium Compounds, Pergamon, Oxford, 1974.
- 16S. V. Pronin, M. G. Tabor, D. J. Janson, R. A. Shenvi, J. Am. Chem. Soc. 2012, 134, 2012–2015.
- 17See the Supporting Information for details.
- 18The kinetic acidity of toluene is known to be higher than that of ethylbenzene. For example, methyl substitution (e.g., toluene→ethylbenzene) has been documented to decrease reactivity with lithium cyclohexylamide base. For a discussion, see: A. Streitwieser, D. E. Van Sickle, J. Am. Chem. Soc. 1962, 84, 249–250.
- 19For a pertinent discussion of the relative rates of carbanion generation from N-methyl versus N-ethyl amides, see: D. R. Hay, Z. Song, S. G. Smith, P. Beak, J. Am. Chem. Soc. 1988, 110, 8145–8153.
- 20The kinetic difficulty in deprotonating methylene positions relative to a methyl position has been applied successfully in directed ortho-metalation chemistry, see: V. Snieckus, Chem. Rev. 1990, 90, 879–933.
- 21The synthesis and purification of isoindolines is known to proceed in low yield, see:
- 21aR. A. Barnes, J. C. Godfrey, J. Org. Chem. 1957, 22, 1038–1043;
- 21bI. Ahmed, G. W. H. Cheeseman, B. Jacques, R. G. Wallace, Tetrahedron 1977, 33, 2255–2258;
- 21cY.-X. Wang, S. Mabic, N. Castagnoli, Jr., Bioorg. Med. Chem. 1998, 6, 143–149;
- 21dD.-R. Hou, M.-S. Wang, M.-W. Chung, Y.-D. Hsieh, H.-H. G. Tsai, J. Org. Chem. 2007, 72, 9231–9239;
- 21eT. Ohmura, A. Kijima, M. Suginome, J. Am. Chem. Soc. 2009, 131, 6070–6071;
- 21fThe mass balance in these reactions was accounted for by nonspecific decomposition products.
- 22Amides have been demonstrated as competent bases for deprotonation of related systems, see:
- 22aR. L. Vaulx, W. H. Puterbaugh, C. R. Hauser, J. Org. Chem. 1964, 29, 3514–3517;
- 22bG. S. Poindexter, J. Org. Chem. 1982, 47, 3787–3788.
- 23In the absence of ZnCl2 and iodine, 24 b is converted into phenanthrylamine i in 49 % yield.
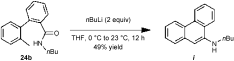
- 24For a recent approach to THNs, as well as a discussion of other synthetic approaches, see: A. K. Mailyan, A. S. Peregudov, P. H. Dixneuf, C. Bruneau, S. N. Osipov, J. Org. Chem. 2012, 77, 8518–8526.
- 25
- 25aY.-X. Cheng, M. Dukat, M. Dowd, W. Fiedler, B. Martin, M. I. Damaj, R. A. Glennon, Eur. J. Med. Chem. 1999, 34, 177–190;
- 25bF. Claudi, G. M. Cingolani, G. Giorgioni, M. Cardellini, F. Amenta, C. Polidori, Eur. J. Med. Chem. 1995, 30, 415–421.
- 26M. R. Del Giudice, C. Mustazza, R. Ferretti, A. Borioni, F. Gatta, J. Heterocycl. Chem. 1998, 35, 915–922.
- 27Ceric ammonium nitrate (CAN) has been applied in related oxidations. See: W.-R. Li, M.-N. Hsu, H.-H. Chou, S. T. Lin, Y.-S. Lin, Chem. Commun. 2000, 401–402.
- 28B. Bachowska, T. Zujewska, ARKIVOC 2001, 77–84.
- 29The starting material (i.e., 32, 6 %) and 5 % of imine 34 was also obtained.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.





