Iridium-Catalyzed Asymmetric Hydrogenation of α-Alkylidene Succinimides†
Yuanyuan Liu
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (China) http://wanbin.sjtu.edu.cn
Search for more papers by this authorCorresponding Author
Prof. Wanbin Zhang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (China) http://wanbin.sjtu.edu.cn
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (China) http://wanbin.sjtu.edu.cn===Search for more papers by this authorYuanyuan Liu
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (China) http://wanbin.sjtu.edu.cn
Search for more papers by this authorCorresponding Author
Prof. Wanbin Zhang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (China) http://wanbin.sjtu.edu.cn
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (China) http://wanbin.sjtu.edu.cn===Search for more papers by this authorThis work was partially supported by the National Natural Science Foundation of China (20972095, 21172143 and 21232004), the Science and Technology Commission of Shanghai Municipality (09JC1407800), Nippon Chemical Industrial Co., Ltd., and Shanghai Jiao Tong University. We thank Prof. Tsuneo Imamoto and Dr. Masashi Sugiya for helpful discussions. Our thanks also go to the Instrumental Analysis Center of Shanghai Jiao Tong University.
Graphical Abstract
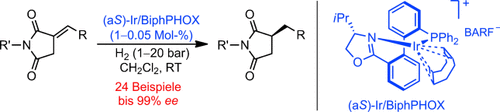
Chirale Succinimid-Derivate sind durch die Titelreaktion mit ausgezeichneten Ausbeuten und Enantioselektivitäten schon bei Zusatz einer geringen Katalysatormenge von 0.05 Mol-% unter milden Bedingungen zugänglich. Wichtige Strukturmotive aus Natur- und Wirkstoffen, namentlich chirale 3-Benzylpyrrolidine und 1-Hydroxypyrrolidin-2,5-dione, konnten problemlos erhalten werden. BARF−=Tetrakis[3,5-bis(trifluormethyl)phenyl]borat.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209126_sm_miscellaneous_information.pdf4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Natural products and pharmaceuticals:
- 1aL. Ducry, B. Stump, H. Wong, J. She, G. Phillips, WO2012113847, 2012;
- 1bL. Iovkova-Berends, C. Wängler, T. Zöller, G. Höfner, K. T. Wanner, C. Rensch, P. Bartenstein, A. Kostikov, R. Schirrmacher, K. Jurkschat, B. Wängler, Molecules 2011, 16, 7458;
- 1cS. C. Bergmeier, K. A. Ismail, K. M. Arason, S. McKay, D. L. Bryant, D. B. McKay, Bioorg. Med. Chem. Lett. 2004, 14, 3739;
- 1dS. G. Davies, D. J. Dixon, J. Chem. Soc. Perkin Trans. 1 1998, 2635;
- 1eA. Fredenhagen, S. Y. Tamura, P. T. M. Kenny, H. Komura, Y. Naya, K. Nakanishi, J. Am. Chem. Soc. 1987, 109, 4409.
- 2Intermediates in organic synthesis:
- 2aE. M. Stang, M. C. White, J. Am. Chem. Soc. 2011, 133, 14892;
- 2bM. A. Berliner, T. Makowski, K. Ng, B. Sitter, C. Wager, Y. Zhang, S. P. A. Dubant, Org. Process Res. Dev. 2011, 15, 1052;
- 2cG. Lunn, B. J. Banks, R. Crook, N. Feeder, A. Pettman, Y. Sabnis, Bioorg. Med. Chem. Lett. 2011, 21, 4608;
- 2dJ. W. Ward, K. Dodd, C. L. Rigby, C. D. Savi, D. J. Dixo, Chem. Commun. 2010, 46, 1691;
- 2eM. Kabata, T. Suzuki, K. Takabe, H. Yoda, Tetrahedron Lett. 2006, 47, 1607;
- 2fH. Okamura, H. Shimizu, Y. Nakamura, T. Iwagawa, M. Nakatani, Tetrahedron Lett. 2000, 41, 4147;
- 2gD. Romo, J. L. Romine, W. Midura, A. I. Meyers, Tetrahedron 1990, 46, 4951;
- 2hM. Y. Kim, J. E. Starrett, Jr., S. M. Weinreb, J. Org. Chem. 1981, 46, 5383.
- 3
- 3aO. Rogel, J.-M. Rondeau, H. Rueeger, O. Simic, F. Sirockin, M. Tintelnot-Blomley, R. Voegeli-Lange, WO2007140980, 2007;
- 3bS. Jolidon, R. Narquizian, R. Norcross, E. Pinard, WO2007147770, 2007;
- 3cW. Breitenstein, S. Cottens, C. Ehrhardt, E. Jacoby, E. L. J. Lorthiois, J. K. Maibaum, N. Ostermann, H. Sellner, O. Simic, WO2006066896, 2006;
- 3dT. A. Chappie, J. M. Humphrey, WO2006070284, 2006;
- 3eR. A. Fairhurst, R. J. Taylor, WO2006097260, 2006;
- 3fC. Thomas, U. Ohnmacht, M. Niger, P. Gmeiner, Bioorg. Med. Chem. Lett. 1998, 8, 2885;
- 3gD. C. Horwell, D. Naylor, H. M. G. Willems, Bioorg. Med. Chem. Lett. 1997, 7, 31;
- 3hG. E. Jagdmann, J. M. Defauw, J. W. Lampe, J. W. Darges, K. Kalter, Bioorg. Med. Chem. Lett. 1996, 6, 1759.
- 4For resent reports on synthesis of racemic succinimides:
- 4aN. Hasegawa, V. Charra, S. Inoue, Y. Fukumoto, N. Chatani, J. Am. Chem. Soc. 2011, 133, 8070;
- 4bE. J. Yoo, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 17378;
- 4cS. Inoue, Y. Fukumoto, N. Chatani, J. Org. Chem. 2007, 72, 6588.
- 5For reviews, see:
- 5aL. M. Stanley, M. P. Sibi, Chem. Rev. 2008, 108, 2887; For selected papers, see:
- 5bX.-F. Xiong, Q. Zhou, J. Gu, L. Dong, T.-Y. Liu, Y.-C. Chen, Angew. Chem. 2012, 124, 4477; Angew. Chem. Int. Ed. 2012, 51, 4401;
- 5cA. Zea, G. Valero, A.-N. R. Alba, A. Moyano, R. Rios, Adv. Synth. Catal. 2010, 352, 1102;
- 5dR. Robles-Machín, I. Alonso, J. Adrio, J. C. Carretero, Chem. Eur. J. 2010, 16, 5286;
- 5eJ. Y.-T. Soh, C.-H. Tan, J. Am. Chem. Soc. 2009, 131, 6904;
- 5fC. Gioia, A. Hauville, L. Bernardi, F. Fini, A. Ricci, Angew. Chem. 2008, 120, 9376;
10.1002/ange.200804275 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9236;
- 5gA. D. Melhado, M. Luparia, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 12638.
- 6
- 6aT. C. Nugent, A. Sadiq, A. Bibi, T. Heine, L. L. Zeonjuk, N. Vankova, B. S. Bassil, Chem. Eur. J. 2012, 18, 4088;
- 6bL. Li, W. Chen, W. Yang, Y. Pan, H. Liu, C.-H. Tan, Z. Jiang, Chem. Commun. 2012, 48, 5124;
- 6cT. Miura, S. Nishida, A. Masuda, N. Tada, A. Itoh, Tetrahedron Lett. 2011, 52, 4158;
- 6dW.-L. Duan, H. Iwamura, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2007, 129, 2130;
- 6eR. Shintani, W.-L. Duan, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 5628.
- 7For reviews, see:
- 7aT. Imamoto in Hydrogenation (Ed.: ), Intech, Rijeka, 2012, Chap. 1, pp. 3–30;
- 7bJ.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713;
- 7cG. Shang, W. Li, X. Zhang in Catalytic Asymmetric Synthesis, 3rd ed. ), Wiley, Hoboken, 2010, pp. 343–436;
- 7d Handbook of Homogeneous Hydrogenation (Eds.: ), Wiley-VCH, Weinheim, 2007;
- 7eW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029;
- 7fR. Noyori, Asymmetric Catalysis in Organic Synthesis, Wiley, New York, 1993.
- 8For reviews, see:
- 8aD. H. Woodmansee, A. Pfaltz, Chem. Commun. 2011, 47, 7912;
- 8bS. J. Roseblade, A. Pfaltz, Acc. Chem. Res. 2007, 40, 1402;
- 8cY.-G. Zhou, Acc. Chem. Res. 2007, 40, 1357;
- 8dK. Källström, I. Munslow, P. G. Andersson, Chem. Eur. J. 2006, 12, 3194;
- 8eX. Cui, K. Burgess, Chem. Rev. 2005, 105, 3272;
- 8fG. Helmchen, A. Pfaltz, Acc. Chem. Res. 2000, 33, 336; For examples of mechanistic studies, see:
- 8gK. H. Hopmann, A. Bayer, Organometallics 2011, 30, 2483;
- 8hC. Mazet, S. P. Smidt, M. Meuwly, A. Pfaltz, J. Am. Chem. Soc. 2004, 126, 14176;
- 8iY. Fan, X. Cui, K. Burgess, M. B. Hall, J. Am. Chem. Soc. 2004, 126, 16688;
- 8jP. Brandt, C. Hedberg, P. G. Andersson, Chem. Eur. J. 2003, 9, 339;
- 8kR. H. Crabtree, P. C. Demou, D. Eden, J. M. Mihelic, C. A. Parnell, J. M. Quirk, G. E. Morris, J. Am. Chem. Soc. 1982, 104, 6994; For selected recent examples, see:
- 8lT. Zhou, B. Peters, M. F. Maldonado, T. Govender, P. G. Andersson, J. Am. Chem. Soc. 2012, 134, 13592;
- 8mS.-F. Zhu, Y.-B. Yu, S. Li, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2012, 124, 9002; Angew. Chem. Int. Ed. 2012, 51, 8872;
- 8nX. Wang, Z. Han, Z. Wang, K. Ding, Angew. Chem. 2012, 124, 960; Angew. Chem. Int. Ed. 2012, 51, 936;
- 8oJ.-H. Xie, X.-Y. Liu, X.-H. Yang, J.-B. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2012, 124, 205; Angew. Chem. Int. Ed. 2012, 51, 201;
- 8pD. H. Woodmansee, M.-A. Müller, L. Tröndlin, E. Hörmann, A. Pfaltz, Chem. Eur. J. 2012, 18, 13780;
- 8qJ.-Q. Li, X. Quan, P. G. Andersson, Chem. Eur. J. 2012, 18, 10609;
- 8rA. Ganić, A. Pfaltz, Chem. Eur. J. 2012, 18, 6724;
- 8sF. W. Patureau, C. Worch, M. A. Siegler, A. L. Spek, C. Bolm, J. N. H. Reek, Adv. Synth. Catal. 2012, 354, 59;
- 8tJ. Mazuela, P.-O. Norrby, P. G. Andersson, O. Pàmies, M. Diéguez, J. Am. Chem. Soc. 2011, 133, 13634;
- 8uJ.-H. Xie, X.-Y. Liu, J.-B. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2011, 123, 7467; Angew. Chem. Int. Ed. 2011, 50, 7329;
- 8vJ. J. Verendel, T. Zhou, J.-Q. Li, A. Paptchikhine, O. Lebedev, P. G. Andersson, J. Am. Chem. Soc. 2010, 132, 8880;
- 8wJ.-B. Xie, J.-H. Xie, X.-Y. Liu, W.-L. Kong, S. Li, Q.-L. Zhou, J. Am. Chem. Soc. 2010, 132, 4538;
- 8xW. Lu, Y. Chen, X. Hou, Angew. Chem. 2008, 120, 10287; Angew. Chem. Int. Ed. 2008, 47, 10133;
- 8yS.-M. Lu, C. Bolm, Angew. Chem. 2008, 120, 9052; Angew. Chem. Int. Ed. 2008, 47, 8920;
- 8zC. Mössner, C. Bolm, Angew. Chem. 2005, 117, 7736;
10.1002/ange.200502482 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7564.
- 9For selected papers on other kinds of ligands used in the iridium-catalyzed hydrogenation, see:
- 9aY. Zhu, K. Burgess, Acc. Chem. Res. 2012, 45, 1623;
- 9bZ.-S. Ye, M.-W. Chen, Q.-A. Chen, L. Shi, Y. Duan, Y.-G. Zhou, Angew. Chem. 2012, 124, 10328; Angew. Chem. Int. Ed. 2012, 51, 10181;
- 9cD. Rageot, D. H. Woodmansee, B. Pugin, A. Pfaltz, Angew. Chem. 2011, 123, 9772;
10.1002/ange.201104105 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9598;
- 9dY. Zhu, Y. Fan, K. Burgess, J. Am. Chem. Soc. 2010, 132, 6249;
- 9eJ. Zhao, K. Burgess, J. Am. Chem. Soc. 2009, 131, 13236;
- 9fG.-H. Hou, J.-H. Xie, P.-C. Yan, Q.-L. Zhou, J. Am. Chem. Soc. 2009, 131, 1366;
- 9gS.-M. Lu, Y.-Q. Wang, X.-W. Han, Y.-G. Zhou, Angew. Chem. 2006, 118, 2318; Angew. Chem. Int. Ed. 2006, 45, 2260;
- 9hM. C. Perry, X. Cui, M. T. Powell, D.-R. Hou, J. H. Reibenspies, K. Burgess, J. Am. Chem. Soc. 2003, 125, 113;
- 9iH.-U. Blaser, Adv. Synth. Catal. 2002, 344, 17;
- 9jD. Xiao, X. Zhang, Angew. Chem. 2001, 113, 3533;
Angew. Chem. Int. Ed. 2001, 40, 3425;
10.1002/1521-3773(20010917)40:18<3425::AID-ANIE3425>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 9kH.-U. Blaser, H.-P. Buser, K. Coers, R. Hanreich, H.-P. Jalett, E. Jelsch, B. Pugin, H.-D. Schneider, F. Spindler, A. Wegmann, Chimia 1999, 53, 275.
- 10
- 10aY. Liu, G. Yang, D. Yao, F. Tian, W. Zhang, Sci. China Ser. B 2011, 54, 87;
- 10bF. Tian, D. Yao, Y. J. Zhang, W. Zhang, Tetrahedron 2009, 65, 9609;
- 10cW. Zhang, F. Xie, H. Yoshinaga, T. Kida, Y. Nakatsuji, I. Ikeda, Synlett 2006, 1185.
- 11
- 11aY. Liu, D. Yao, K. Li, F. Tian, F. Xie, W. Zhang, Tetrahedron 2011, 67, 8445;
- 11bF. Tian, D. Yao, Y. Liu, W. Zhang, Adv. Synth. Catal. 2010, 352, 1841.
- 12aY. Imai, W. Zhang, T. Kida, Y. Nakatsuji, I. Ikeda, Tetrahedron Lett. 1998, 39, 4343;
- 12bM. Ogasawara, K. Yoshida, H. Kamei, K. Kato, Y. Uozumi, T. Hayashi, Tetrahedron: Asymmetry 1998, 9, 1779;
- 12cD. Liu, F. Xie, W. Zhang, Tetrahedron Lett. 2007, 48, 7591.
- 13I. Ebashi, T. Takigawa, M. Inoue, US005336782A, 1994.
- 14W. C. Groutas, M. J. Brubaker, M. A. Stanga, J. C. Castrisos, J. P. Crowley, E. J. Schatz, J. Med. Chem. 1989, 32, 1607.
- 15CCDC 762042 [(aS)-Ir/iPr-BiphPHOX] contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 16Previous studies showed that the CC bond coordinated with iridium at the oxazoline side. Please see Refs. [8g–k].
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.