Silver-Catalyzed Hydrotrifluoromethylation of Unactivated Alkenes with CF3SiMe3†
Xinyue Wu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)
Search for more papers by this authorDr. Lingling Chu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng-Ling Qing
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)
College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620 (China)
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)===Search for more papers by this authorXinyue Wu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)
Search for more papers by this authorDr. Lingling Chu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng-Ling Qing
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)
College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620 (China)
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 (China)===Search for more papers by this authorThis work was supported by the National Natural Science Foundation of China (21072028, 21272036) and the National Basic Research Program of China (2012CB21600).
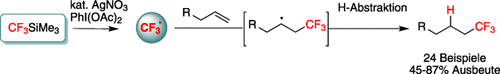
Graphical Abstract
Zwischenzeitlich radikal: Die Titelreaktion führt zur selektiven Bildung trifluormethylierter Alkane und ist komplementär zur bekannten übergangsmetallkatalysierten Trifluormethylierung von Olefinen, die trifluormethylierte allylische Verbindungen ergibt. Mechanistische Studien deuten an, dass die Hydrotrifluormethylierung über ein CF3-Radikal verläuft.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201208971_sm_miscellaneous_information.pdf4.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Kirsch, Modern Fluoroorganic Chemistry; Wiley-VCH, Weinheim, 2004;
10.1002/352760393X Google Scholar
- 1bK. Muller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 1cM. Hird, Chem. Soc. Rev. 2007, 36, 2070;
- 1dK. L. Kirk, Org. Process Res. Dev. 2008, 12, 305.
- 2
- 2aM. Schlosser, Angew. Chem. 2006, 118, 5558;
10.1002/ange.200600449 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5432;
- 2bJ.-A. Ma, D. Cahard, J. Fluorine Chem. 2007, 128, 975;
- 2cJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1;
- 2dA. Studer, Angew. Chem. 2012, 124, 9082; Angew. Chem. Int. Ed. 2012, 51, 8950.
- 3For recent reviews on transition-metal-mediated trifluoromethylation, see:
- 3aM. A. McClinton, D. A. McClinton, Tetrahedron 1992, 48, 6555;
- 3bS. Roy, B. T. Gregg, G. W. Gribble, V.-D. Le, S. Roy, Tetrahedron 2011, 67, 2161;
- 3cO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475;
- 3dT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470;
- 3eY. Ye, M. S. Sanford, Synlett 2012, 2005;
- 3fX.-F. Wu, H. Neumann, M. Beller, Chem. Asian J. 2012, 7, 1744;
- 3gT. Besset, C. Schneider, D. Cahard, Angew. Chem. 2012, 124, 5134;
10.1002/ange.201201012 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5048;
- 3hF. Pan, Z. Shi, Acta Chim. Sin. 2012, 70, 1679;
- 3iF.-L. Qing, Chin. J. Org. Chem. 2012, 32, 815;
- 3jC. Lü, Q. Shen, D. Liu, Chin. J. Org. Chem. 2012, 32, 1380;
- 3kT. Liu, Q. Shen, Eur. J. Org. Chem. 2012, 6679.
- 4For palladium-mediated trifluoromethylation, see:
- 4aV. V. Grushin, W. J. Marshall, J. Am. Chem. Soc. 2006, 128, 4632;
- 4bV. V. Grushin, W. J. Marshall, J. Am. Chem. Soc. 2006, 128, 12644;
- 4cN. D. Ball, J. W. Kampf, M. S. Sanford, J. Am. Chem. Soc. 2010, 132, 2878;
- 4dY. Ye, N. D. Ball, J. W. Kampf, M. S. Sanford, J. Am. Chem. Soc. 2010, 132, 14682;
- 4eN. D. Ball, J. B. Gary, Y. Ye, M. S. Sanford, J. Am. Chem. Soc. 2011, 133, 7577.
- 5For palladium-catalyzed trifluoromethylation, see:
- 5aX. Wang, L. Truesdale, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 3648;
- 5bE. J. Cho, T. D. Senecal, T. Kinzel, Y. Zhang, D. A. Watson, S. L. Buchwald, Science 2010, 328, 1679;
- 5cX. Mu, S. Chen, X. Zhen, G. Liu, Chem. Eur. J. 2011, 17, 6039;
- 5dB. S. Samant, G. W. Kabalka, Chem. Commun. 2011, 47, 7236;
- 5eR. N. Loy, M. S. Sanford, Org. Lett. 2011, 13, 2548;
- 5fX. Mu, T. Wu, H.-y. Wang, Y.-l. Guo, G. Liu, J. Am. Chem. Soc. 2012, 134, 878;
- 5gX.-G. Zhang, H.-X. Dai, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2012, 134, 11948.
- 6For copper-mediated trifluoromethylation, see:
- 6aG. G. Dubinina, H. Furutachi, D. A. Vicic, J. Am. Chem. Soc. 2008, 130, 8600;
- 6bL. Chu, F.-L. Qing, Org. Lett. 2010, 12, 5060;
- 6cT. D. Senecal, A. T. Parsons, S. L. Buchwald, J. Org. Chem. 2011, 76, 1174;
- 6dC.-P. Zhang, Z.-L. Wang, Q.-Y. Chen, C.-T. Zhang, Y.-C. Gu, J.-C. Xiao, Angew. Chem. 2011, 123, 1936; Angew. Chem. Int. Ed. 2011, 50, 1896;
- 6eH. Morimoto, T. Tsubogo, N. D. Litvinas, J. F. Hartwig, Angew. Chem. 2011, 123, 3877;
10.1002/ange.201100633 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3793;
- 6fO. A. Tomashenko, E. C. Escudero-Adan, M. M. Belmonte, V. V. Grushin, Angew. Chem. 2011, 123, 7797;
10.1002/ange.201101577 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7655;
- 6gA. Zanardi, M. A. Novikov, E. Martin, J. Benet-Buchholz, V. V. Grushin, J. Am. Chem. Soc. 2011, 133, 20901;
- 6hN. D. Litvinas, P. S. Fier, J. F. Hartwig, Angew. Chem. 2012, 124, 551;
10.1002/ange.201106668 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 536;
- 6iB. A. Khan, A. E. Buba, L. J. Gooßen, Chem. Eur. J. 2012, 18, 1577;
- 6jP. Novák, A. Lishchynskyi, V. V. Grushin, Angew. Chem. 2012, 124, 7887; Angew. Chem. Int. Ed. 2012, 51, 7767;
- 6kY. Ye, S. Kunzi, M. S. Sanford, Org. Lett. 2012, 14, 4979;
- 6lM. Hu, C. Ni, J. Hu, J. Am. Chem. Soc. 2012, 134, 15257.
- 7For copepr-catalyzed trifluoromethylation, see:
- 7aM. Oishi, H. Kondo, H. Amii, Chem. Commun. 2009, 1909;
- 7bT. Knauber, F. Arikan, G.-V. Röschenthaler, L. J. Gooßen, Chem. Eur. J. 2011, 17, 2689;
- 7cZ. Weng, R. Lee, W. Jia, Y. Yuan, W. Wang, X. Feng, K.-W. Huang, Organometallics 2011, 30, 3229;
- 7dJ. Xu, D.-F. Luo, B. Xiao, Z.-J. Liu, T.-J. Gong, Y. Fu, L. Liu, Chem. Commun. 2011, 47, 4300;
- 7eT. Liu, Q. Shen, Org. Lett. 2011, 13, 2342;
- 7fT. Liu, X. Shao, Y. Wu, Q. Shen, Angew. Chem. 2012, 124, 555; Angew. Chem. Int. Ed. 2012, 51, 540;
- 7gL. Chu, F.-L. Qing, J. Am. Chem. Soc. 2012, 134, 1298;
- 7hX. L. Jiang, L. Chu, F.-L. Qing, J. Org. Chem. 2012, 77, 1251;
- 7iY. Huang, X. Fang, X. Lin, H. Li, W. He, K. W. Huang, Y. Yuan, Z. Weng, Tetrahedron 2012, 68, 9949;
- 7jY. Miyake, S. Ota, Y. Nishibayashi, Chem. Eur. J. 2012, 18, 13255;
- 7kY. Ye, M. S. Sanford, J. Am. Chem. Soc. 2012, 134, 9034;
- 7lD. Luo, J. Xu, Y. Fu, Q. X. Guo, Tetrahedron Lett. 2012, 53, 2769.
- 8
- 8aY. Ye, S. H. Lee, M. S. Sanford, Org. Lett. 2011, 13, 5464;
- 8bA. Hafner, S. Bräse, Angew. Chem. 2012, 124, 3773;
10.1002/ange.201107414 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3713.
- 9
- 9aM. Naodovic, H. Yamamoto, Chem. Rev. 2008, 108, 3132;
- 9bJ.-M. Weibel, A. L. Blanc, P. Pale, Chem. Rev. 2008, 108, 3149.
- 10
- 10aT. Furuya, A. E. Strom, T. Ritter, J. Am. Chem. Soc. 2009, 131, 1662;
- 10bT. Furuya, T. Ritter, Org. Lett. 2009, 11, 2860;
- 10cP. Tang, T. Furuya, T. Ritter, J. Am. Chem. Soc. 2010, 132, 12150;
- 10dT. Furuya, J. E. M. N. Klein, T. Ritter, Synthesis 2010, 1804;
- 10eP. Tang, T. Ritter, Tetrahedron 2011, 67, 4449;
- 10fF. Yin, Z. Wang, Z. Li, C. Li, J. Am. Chem. Soc. 2012, 134, 10401;
- 10gT. Xu, G. Liu, Synlett 2012, 955.
- 11G. Teverovskiy, D. S. Surry, S. L. Buchwald, Angew. Chem. 2011, 123, 7450;
10.1002/ange.201102543 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7312.
- 12C. Huang, T. Liang, S. Harada, E. Lee, T. Ritter, J. Am. Chem. Soc. 2011, 133, 13308.
- 13
- 13aA. T. Parsons, S. L. Buchwald, Angew. Chem. 2011, 123, 9286; Angew. Chem. Int. Ed. 2011, 50, 9120;
- 13bX. Wang, Y. Ye, S. Zhang, J. Feng, Y. Xu, Y. Zhang, J. Wang, J. Am. Chem. Soc. 2011, 133, 16410;
- 13cJ. Xu, Y. Fu, D. F. Luo, Y. Y. Jiang, B. Xiao, Z. J. Liu, T. J. Gong, L. Liu, J. Am. Chem. Soc. 2011, 133, 15300;
- 13dR. Shimizu, H. Egami, Y. Hamashima, M. Sodeoka, Angew. Chem. 2012, 124, 4655; Angew. Chem. Int. Ed. 2012, 51, 4577;
- 13eS. Mizuta, O. Galicia-López, K. M. Engle, S. Verhoog, K. Wheelhouse, G. Rassias, V. Gouverneur, Chem. Eur. J. 2012, 18, 8583.
- 14
- 14aP. G. Janson, I. Ghoneim, N. O. Ilchenko, K. J. Szabó, Org. Lett. 2012, 14, 2882;
- 14bY. Li, A. Studer, Angew. Chem. 2012, 124, 8345; Angew. Chem. Int. Ed. 2012, 51, 8221;
- 14cY. Yasu, T. Koike, M. Akita, Angew. Chem. 2012, 124, 9705;
10.1002/ange.201205071 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9567;
- 14dH. Egami, R. Shimizu, M. Sodeoka, Tetrahedron Lett. 2012, 53, 5503;
- 14eR. Zhu, S. L. Buchwald, J. Am. Chem. Soc. 2012, 134, 12462;
- 14fC. Feng, T.-P. Loh, Chem. Sci. 2012, 3, 3458.
- 15L. Chu, F.-L. Qing, Org. Lett. 2012, 14, 2106.
- 16Electrochemical hydrotrifluoromethylation, see:
- 16aN. Muller, J. Org. Chem. 1986, 51, 263;
- 16bN. Muller, J. Fluorine Chem. 1987, 36, 163;
- 16cK. Uneyama, S. Watanbe, J. Org. Chem. 1990, 55, 3909;
- 16dK. Uneyama, S. Watanabe, Y. Tokunaga, K. Kitagawa, Y. Sato, Bull. Chem. Soc. Jpn. 1992, 65, 1976;
- 16eY. Dan-oh, K. Uneyama, Bull. Chem. Soc. Jpn. 1995, 68, 2993;
- 16fJ.-B. Tommasino, A. Brondex, M. Médebielle, M. Thomalla, B. R. Langlois, T. Billard, Synlett 2002, 1697;
- 16gP. Wang, Y. Tang, D. A. Tirrell, J. Am. Chem. Soc. 2003, 125, 6900.
- 17For hydrotrifluoromethylation of α,β-unsaturated ketones or acyl-oxazolidiones, see:
- 17aK. Sato, M. Omote, A. Ando, I. Kumadaki, Org. Lett. 2004, 6, 4359;
- 17bH. Erdbrink, I. Peuser, U. I. M. Gerling, D. Lentz, B. Koksch, C. Czekelius, Org. Biomol. Chem. 2012, 10, 8583.
- 18
- 18aK. Maruoka, H. Sano, Y. Fukutani, H. Yamamoto, Chem. Lett. 1985, 1689;
- 18bJ. Ignatowska, W. Dmowski, J. Fluorine Chem. 2007, 128, 997.
- 19
- 19aM. Hanack, H. Meyer, Justus Liebigs Ann. Chem. 1968, 720, 81;
- 19bZ. Liu, K. Kumar, Synthesis 2010, 1905.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.