Facile Carbon Monoxide Reduction at Intramolecular Frustrated Phosphane/Borane Lewis Pair Templates†
Dr. Muhammad Sajid
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorLisa-Maria Elmer
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorChristoph Rosorius
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorProf. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstraße 4, 53115 Bonn (Germany)
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)===Search for more papers by this authorDr. Muhammad Sajid
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorLisa-Maria Elmer
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorChristoph Rosorius
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorProf. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstraße 4, 53115 Bonn (Germany)
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)
Organisch-Chemisches Institut der Universität Münster, Corrensstraße 40, 48149 Münster (Germany)===Search for more papers by this authorFinancial Support from the European Research Council and the Deutsche Forschungsgemeinschaft is gratefully acknowledged.
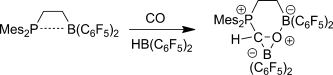
Graphical Abstract
Ein neuer Reaktionspfad: Kohlenmonoxid wird leicht mit Piers' Boran und einem frustrierten Lewis-Paar (FLP) zu einem FLP-stabilisierten Formylboran reduziert (siehe Bild). Diese Reaktion kann als ein typisches Beispiel für die effiziente Aktivierung eines kleinen Moleküls durch ein FLP betrachtet werden.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201208750_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. C. Brown, Acc. Chem. Res. 1969, 2, 65–72.
- 2
- 2aM. E. D. Hillman, J. Am. Chem. Soc. 1963, 85, 1626–1628;
- 2bM. E. D. Hillman, J. Am. Chem. Soc. 1963, 85, 982–984; see also:
- 2cA. Pelter, M. G. Hutchings, K. Smith, D. J. Williams, J. Chem. Soc. Perkin Trans. 1 1974, 145–150;
- 2dA. Pelter, K. Smith, M. G. Hutchings, K. Rowe, J. Chem. Soc. Perkin Trans. 1 1975, 2, 129–138;
- 2eG. W. Kabalka, J. T. Gotsick, R. D. Pace, N.-S. Li, Organometallics 1994, 13, 5163–5165;
- 2fV. K. Aggarwal, G. Y. Fang, X. Ginesta, D. M. Howells, M. Zaja, Pure Appl. Chem. 2006, 78, 215–229;
- 2gM. A. Dureen, D. W. Stephan, J. Am. Chem. Soc. 2010, 132, 13559–13568.
- 3
- 3aT. W. Bentley, J. Org. Chem. 1982, 47, 60–64; see also:
- 3bE. Kaufmann, P. v. Ragué Schleyer, S. Gronert, A. Streitwieser Jr., M. Halpern, J. Am. Chem. Soc. 1987, 109, 2553–2559; P. v. Ragué Schleyer, S. Gronert, A. Streitwieser Jr., M. Halpern, J. Am. Chem. Soc. 1987, 109, 2553–2559;
- 3cM. Yalpani, R. Köster, J. Organomet. Chem. 1992, 434, 133–141.
- 4This is similar to the reluctance of direct insertion of CO into the ZrH bonds of many d0 ZrIV hydrido complexes, see e.g.:
- 4aJ. M. Manriquez, D. R. McAlister, R. D. Sanner, J. E. Bercaw, J. Am. Chem. Soc. 1978, 100, 2716–2724;
- 4bH. Berke, R. Hoffmann, J. Am. Chem. Soc. 1978, 100, 7224–7236;
- 4cT. Wolczanski, R. S. Threlkel, J. E. Bercaw, J. Am. Chem. Soc. 1979, 101, 218–220;
- 4dT. Wolczanski, J. E. Bercaw, J. Am. Chem. Soc. 1979, 101, 6450–6452;
- 4eP. T. Wolczanski, J. E. Bercaw, Acc. Chem. Res. 1980, 13, 121–127;
- 4fG. Erker, K. Kropp, C. Krüger, A.-P. Chiang, Chem. Ber. 1982, 115, 2447–2460;
- 4gK. Kropp, V. Skibbe, G. Erker, C. Krüger, J. Am. Chem. Soc. 1983, 105, 3353–3354;
- 4hG. Erker, Acc. Chem. Res. 1984, 17, 103–109; see also:
- 4iA. Berkefeld, W. E. Piers, M. Parvez, L. Castro, L. Maron, O. Eisenstein, J. Am. Chem. Soc. 2012, 134, 10843–10851.
- 5A. G. Burg, H. I. Schlesinger, J. Am. Chem. Soc. 1937, 59, 780–787.
- 6
- 6aT. P. Fehlner, W. S. Koski, J. Am. Chem. Soc. 1965, 87, 409–413;
- 6bJ. C. Carter, A. L. Moyé, G. W. Luther III, J. Am. Chem. Soc. 1974, 96, 3071–3073;
- 6cH. Umeyama, K. Morokuma, J. Am. Chem. Soc. 1976, 98, 7208–7220;
- 6dR. G. Montemayor, R. W. Parry, Inorg. Chem. 1979, 18, 1470–1473;
- 6eB. F. Spielvogel, A. T. McPhail, J. A. Knight, C. G. Moreland, C. L. Gatchell, K. W. Morse, Polyhedron 1983, 2, 1345–1352;
- 6fA. S. Goldman, K. Krogh-Jespersen, J. Am. Chem. Soc. 1996, 118, 12159–12166;
- 6gC. M. Álvarez, R. Carrillo, R. García-Rudríguez, D. Miguel, Chem. Commun. 2012, 48, 7705–7707;
- 6hA. Fukazawa, J. L. Dutton, C. Fan, L. G. Mercier, A. Y. Houghton, Q. Wu, W. E. Piers, M. Parvez, Chem. Sci. 2012, 3, 1814–1818.
- 7D. W. Stephan, G. Erker, Angew. Chem. 2010, 122, 50–81; Angew. Chem. Int. Ed. 2010, 49, 46–76.
- 8A. E. Ashley, D. O’Hare, Top. Curr. Chem. 2012, DOI: , and references therein.
- 9
- 9aD. J. Parks, R. E. von H. Spence, W. E. Piers, Angew. Chem. 1995, 107, 895–897;
10.1002/ange.19951070724 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 809–811;
- 9bR. E. von H. Spence, D. J. Parks, W. E. Piers, M.-A. MacDonald, M. J. Zaworotko, S. J. Rettig, Angew. Chem. 1995, 107, 1337–1340;
10.1002/ange.19951071118 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1230–1233; Review:
- 9cW. E. Piers, T. Chivers, Chem. Soc. Rev. 1997, 26, 345–354;
- 9dD. J. Parks, W. E. Piers, G. P. A. Yap, Organometallics 1998, 17, 5459–5503.
- 10
- 10aP. Spies, G. Erker, G. Kehr, K. Bergander, R. Fröhlich, S. Grimme, D. W. Stephan, Chem. Commun. 2007, 5072–5074;
- 10bG. Kehr, S. Schwendemann, G. Erker, Top. Curr. Chem. 2012, DOI: .
- 11See for a comparison:
- 11aK. Axenov, C. M. Mömming, G. Kehr, R. Fröhlich, G. Erker, Chem. Eur. J. 2010, 16, 14069–14073;
- 11bS. Schwendemann, R. Fröhlich, G. Kehr, G. Erker, Chem. Sci. 2011, 2, 1842–1849.
- 12
- 12aV. N. Staroverov, G. E. Scuseria, J. Tao, J. P. Perdew, J. Chem. Phys. 2003, 119, 12129–12137;
- 12bY. Zhao, D. G. Truhlar, J. Phys. Chem. A 2005, 109, 5656–5667;
- 12cF. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297–3305;
- 12dS. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104–154119;
- 12eS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465;
- 12fK. Eichkorn, F. Weigend, O. Treutler, R. Ahlrichs, Theor. Chem. Acc. 1997, 97, 119–124;
- 12gS. Grimme, Chem. Eur. J. 2012, 18, 9955–9964;
- 12hA. Klamt, WIREs Comput. Mol. Sci. 2011, 1, 699–709.
- 13
- 13aC. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan, G. Erker, Angew. Chem. 2009, 121, 6770–6773;
10.1002/ange.200901636 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6643–6646;
- 13bR. C. Neu, E. Otten, A. Lough, D. W. Stephan, Chem. Sci. 2011, 2, 170–176;
- 13cA. J. P. Cardenas, B. J. Culotta, T. H. Warren, S. Grimme, A. Stute, R. Fröhlich, G. Kehr, G. Erker, Angew. Chem. 2011, 123, 7709–7713;
10.1002/ange.201101622 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7567–7571;
- 13dM. Sajid, A. Stute, A. J. P. Cardenas, B. J. Culotta, J. A. M. Hepperle, T. H. Warren, B. Schirmer, S. Grimme, A. Studer, C. G. Daniliuc, R. Fröhlich, J. L. Petersen, G. Kehr, G. Erker, J. Am. Chem. Soc. 2012, 134, 10156–10168;
- 13eM. Sajid, A. Klose, B. Birkmann, L. Liang, B. Schirmer, T. Wiegand, H. Eckert, A. J. Lough, R. Fröhlich, C. G. Daniliuc, S. Grimme, D. W. Stephan, G. Kehr, G. Erker, Chem. Sci. 2013, 4, 213–219.
- 14One can easily formulate alternative CO reduction pathways. A referee suggested potential carbon monoxide reduction at a preformed FLP–CO adduct as an alternative. We have found indications that both the FLPs 4 and 6 indeed form CO adducts, but both are thermally rather unstable and loose the CO molecule above −20 °C (M. Sajid, W. Dong, G. Kehr, G. Erker, unpublished).
- 15CCDC 916134 (2), CCDC 916135 (5), and CCDC 916136 (7) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.