Relay Catalytic Branching Cascade: A Technique to Access Diverse Molecular Scaffolds†
Corresponding Author
Dr. Nitin T. Patil
Division of Crop Protection Chemicals, CSIR—Indian Institute of Chemical Technology, Hyderabad—500007 (India)
Division of Crop Protection Chemicals, CSIR—Indian Institute of Chemical Technology, Hyderabad—500007 (India)===Search for more papers by this authorValmik S. Shinde
Division of Crop Protection Chemicals, CSIR—Indian Institute of Chemical Technology, Hyderabad—500007 (India)
Search for more papers by this authorDr. Balasubramanian Sridhar
Laboratory of X-Ray Crystallography, CSIR—Indian Institute of Chemical Technology, Hyderabad–500007 (India)
Search for more papers by this authorCorresponding Author
Dr. Nitin T. Patil
Division of Crop Protection Chemicals, CSIR—Indian Institute of Chemical Technology, Hyderabad—500007 (India)
Division of Crop Protection Chemicals, CSIR—Indian Institute of Chemical Technology, Hyderabad—500007 (India)===Search for more papers by this authorValmik S. Shinde
Division of Crop Protection Chemicals, CSIR—Indian Institute of Chemical Technology, Hyderabad—500007 (India)
Search for more papers by this authorDr. Balasubramanian Sridhar
Laboratory of X-Ray Crystallography, CSIR—Indian Institute of Chemical Technology, Hyderabad–500007 (India)
Search for more papers by this authorGenerous financial support by the Department of Science and Technology (DST) and the Council of Scientific and Industrial Research (CSIR), New Delhi, India, is gratefully acknowledged. V.S.S. thanks the UGC for a senior research fellowship.
Graphical Abstract
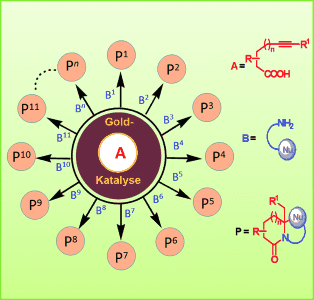
Gerüstvielfalt: Die Gold-katalysierten Reaktionen von Alkinsäuren (A) mit verschiedenen gerüstgebenden Substraten (B) führen zur Bildung einer großen Zahl multifunktioneller polyheterocyclischer Strukturen (siehe Schema). Dieser Ansatz ermöglicht die Herstellung von Substanzbibliotheken mit hoher Gerüstdiversität.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201208738_sm_miscellaneous_information.pdf14.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. R. J. D. Galloway, M. Diáz-Gavilán, A. Isidro-Llobet, D. R. Spring, Angew. Chem. 2009, 121, 1216–1218;
10.1002/ange.200805452 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1194–1196.
- 2S. L. Schreiber, Science 2000, 287, 1964–1969.
- 3Reviews:
- 3aA. Tavassoli, A. D. Hamilton, D. R. Spring, Chem. Soc. Rev. 2011, 40, 4269–4270;
- 3bT. W. J. Cooper, I. B. Campbell, S. J. F. Macdonald, Angew. Chem. 2010, 122, 8258–8267;
10.1002/ange.201002238 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8082–8091;
- 3cW. R. J. D. Galloway, A. Isidro-Llobet, D. R. Spring, Nat. Commun. 2010, 1, 80;
- 3dK. Grabowski, K.-H. Baringhaus, G. Schneider, Nat. Prod. Rep. 2008, 25, 892–904;
- 3eM. Kaiser, S. Wetzel, K. Kumar, H. Waldmann, Cell. Mol. Life Sci. 2008, 65, 1186–1201;
- 3fD. R. Spring, Chem. Soc. Rev. 2005, 34, 472–482;
- 3gC. M. Dobson, Nature 2004, 432, 824–828;
- 3hB. R. Stockwell, Nature 2004, 432, 846–854;
- 3iC. Lipinski, A. Hopkins, Nature 2004, 432, 855–861.
- 4
- 4aE. Ascic, S. T. Le Quement, M. Ishoey, M. Daugaard, T. E. Nielsen, ACS Comb. Sci. 2012, 14, 253–257;
- 4bT. Luo, S. L. Schreiber, J. Am. Chem. Soc. 2009, 131, 5667–5674;
- 4cT. E. Nielsen, S. L. Schreiber, Angew. Chem. 2008, 120, 52–61;
10.1002/ange.200703073 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 48–56.
- 5
- 5aQ. Zang, S. Javed, F. Ullah, A. Zhou, C. A. Knudtson, D. Bi, F. Z. Basha, M. G. Organ, P. R. Hanson, Synthesis 2011, 2743–2750;
- 5bA. Rolfe, G. H. Lushington, P. R. Hanson, Org. Biomol. Chem. 2010, 8, 2198–2203.
- 6
- 6aA. W. Hung, A. Ramek, Y. Wang, T. Kaya, J. A. Wilson, P. A. Clemons, D. W. Young, Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804;
- 6bC. W. Murray, D. C. Rees, Nat. Chem. 2009, 1, 187–192;
- 6cP. J. Hajduk, J. A. Greer, Nat. Rev. Drug Discovery 2007, 6, 211–219.
- 7S. Dandapani, L. A. Marcaurelle, Curr. Opin. Chem. Biol. 2010, 14, 362–370.
- 8
- 8aE. E. Wyatt, S. Fergus, W. R. J. D. Galloway, A. Bender, D. J. Fox, A. T. Plowright, A. S. Jessiman, D. R. Spring, Chem. Commun. 2006, 3296–3298;
- 8bO. Kwon, S. B. Park, S. L. Schreiber, J. Am. Chem. Soc. 2002, 124, 13402–13404.
- 9W. Liu, V. Khedkar, B. Baskar, M. Schümann, K. Kumar, Angew. Chem. 2011, 123, 7032–7037; Angew. Chem. Int. Ed. 2011, 50, 6900–6905.
- 10D. Robbins, A. F. Newton, C. Gignoux, J.-C. Legeay, A. Sinclair, M. Rejzek, C. A. Laxon, S. K. Yalamanchili, W. Lewis, M. A. O’Connell, R. A. Stockman, Chem. Sci. 2011, 2, 2232–2235.
- 11
- 11aD. Morton, S. Leach, C. Cordier, S. Warriner, A. Nelson, Angew. Chem. 2009, 121, 110–115; Angew. Chem. Int. Ed. 2009, 48, 104–109;
- 11bG. L. Thomas, R. J. Spandl, F. G. Glansdorp, M. Welch, A. Bender, J. Cockfield, J. A. Lindsay, C. Bryant, D. F. J. Brown, O. Loiseleur, H. Rudyk, M. Ladlow, D. R. Spring, Angew. Chem. 2008, 120, 2850–2854; Angew. Chem. Int. Ed. 2008, 47, 2808–2812.
- 12M. D. Burke, E. M. Berger, S. L. Schreiber, Science 2003, 302, 613–618.
- 13For reports on catalytic approaches to multifunctional polyheterocyclic from our laboratory, see:
- 13aN. T. Patil, V. S. Raut, V. S. Shinde, G. Gayatri, G. N. Sastry, Chem. Eur. J. 2012, 18, 5530–5535;
- 13bN. T. Patil, A. K. Mutyala, A. Konala, R. B. Tella, Chem. Commun. 2012, 48, 3094–3096;
- 13cN. T. Patil, P. G. V. V. Lakshmi, B. Sridhar, S. Patra, M. P. Bhadra, C. R. Patra, Eur. J. Org. Chem. 2012, 1790–1799;
- 13dN. T. Patil, V. Singh, Chem. Commun. 2011, 47, 11116–11118;
- 13eN. T. Patil, V. S. Raut, J. Org. Chem. 2010, 75, 6961–6964;
- 13fN. T. Patil, P. G. V. V. Lakshmi, V. Singh, Eur. J. Org. Chem. 2010, 4719–4731;
- 13gN. T. Patil, R. D. Kavthe, V. S. Shinde, B. Sridhar, J. Org. Chem. 2010, 75, 3371–3380;
- 13hN. T. Patil, A. K. Mutyala, P. G. V. V. Lakshmi, P. V. K. Raju, B. Sridhar, Eur. J. Org. Chem. 2010, 1999–2007;
- 13iN. T. Patil, R. D. Kavthe, V. S. Raut, V. S. Shinde, B. Sridhar, J. Org. Chem. 2010, 75, 1277–1280;
- 13jN. T. Patil, A. Konala, Eur. J. Org. Chem. 2010, 6831–6839;
- 13kN. T. Patil, R. D. Kavthe, V. S. Raut, V. V. N. Reddy, J. Org. Chem. 2009, 74, 6315–6318.
- 14
- 14aN. T. Patil, A. K. Mutyala, P. G. V. V. Lakshmi, B. Gajula, B. Sridhar, G. R. Pottireddygari, T. P. Rao, J. Org. Chem. 2010, 75, 5963–5975;
- 14bT. Yang, L. Campbell, D. J. Dixon, J. Am. Chem. Soc. 2007, 129, 12070–12071.
- 15N. T. Patil, V. S. Shinde, B. Gajula, Org. Biomol. Chem. 2012, 10, 211–224.
- 16
- 16aA. S. K. Hashmi, M. Rudolph, Chem. Soc. Rev. 2008, 37, 1766–1775;
- 16bE. Jiménez-Núñez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326–3350;
- 16cA. Arcadi, Chem. Rev. 2008, 108, 3266–3325;
- 16dZ. Li, C. Brouwer, C. He, Chem. Rev. 2008, 108, 3239–3265;
- 16eD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351–3378;
- 16fS. Md. Abu Sohel, R.-S. Liu, Chem. Soc. Rev. 2009, 38, 2269–2281;
- 16gA. S. Dudnik, N. Chernyak, V. Gevorgyan, Aldrichimica Acta 2010, 43, 37–46;
- 16hM. Bandini, Chem. Soc. Rev. 2011, 40, 1358–1367;
- 16iN. T. Patil, V. Singh, J. Organomet. Chem. 2011, 696, 419–432;
- 16jN. T. Patil, Chem. Asian J. 2012, 7, 2186–2194.
- 17CCDC 897931 (6 b), 897934 (12 a), 897932 (14 b), 897929 (16 a), 897930 (18 a), 897933 (20 a) and 897928 (28 c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif..
- 18See the Supporting Information for a detailed list of reports on the synthesis and biological importance of all scaffolds.
- 19
- 19aJ. H. Kalin, K. V. Butler, T. Akimova, W. W. Hancock, A. P. Kozikowski, J. Med. Chem. 2012, 55, 639–651;
- 19bJ. Bonjoch, F. Diaba, L. Pagès, D. Pérez, L. Soca, M. Miralpeix, D. Vilella, P. Anton, C. Puig, Bioorg. Med. Chem. Lett. 2009, 19, 4299–4302;
- 19cN. Khorana, A, Purohit, K. Herrick-Davis, M. Teitler, R. A. Glennon, Bioorg. Med. Chem. 2003, 11, 717–722.
- 20R. Grigg, V. Sridharan, D. A. Sykes, Tetrahedron 2008, 64, 8952–8962.
- 21X.-Y. Liu, C.-M. Che, Angew. Chem. 2008, 120, 3865–3870; Angew. Chem. Int. Ed. 2008, 47, 3805–3810.
- 22
- 22aD. C. Oniciu, J- ;L. Henri, R. Barbaras, V. Kochubey, D. Kovalsky, O. G. Rodin, O. Geoffroy, A. Rzepiela, (Cerenis Therapeutics SA), WO 2012/054535A2, 2012;
- 22bN. Khorana, C. Smith, K. Herrick-Davis, A. Purohit, M. Teitler, B. Grella, M. Dukat, R. A. Glennon, J. Med. Chem. 2003, 46, 3930–3937;
- 22cC. Altomare, L. Summo, S. Cellamare, A. V. Varlamov, L. G. Voskressensky, T. N. Borisova, A. Carotti, Bioorg. Med. Chem. Lett. 2000, 10, 581–584.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.