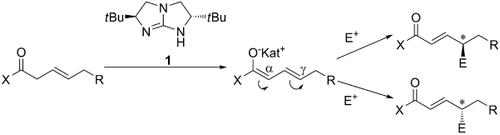
Enantiodivergent and γ-Selective Asymmetric Allylic Amination†
Jianmin Wang
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
These authors contributed equally to this work.
Search for more papers by this authorJie Chen
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
These authors contributed equally to this work.
Search for more papers by this authorChoon Wee Kee
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Choon-Hong Tan
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)Search for more papers by this authorJianmin Wang
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
These authors contributed equally to this work.
Search for more papers by this authorJie Chen
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
These authors contributed equally to this work.
Search for more papers by this authorChoon Wee Kee
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Choon-Hong Tan
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)Search for more papers by this authorThis work was supported by ARF grant R-143-000-461-112 and scholarships (to J.M. and C.W.K) from the National University of Singapore.
Graphical Abstract
Doppelagent: Die Titelreaktion mit dem Guanidinkatalysator 1 kann beide Produkt-Enantiomere mit ausgezeichneten Enantioselektivitäten liefern, wenn die β,γ-ungesättigte Carbonylverbindung mit der richtigen Doppelbindungskonfiguration eingesetzt wird. Rechnungen zeigen eine mögliche Ursache dieser invertierten Enantioselektivität. Das Verfahren ist vielversprechend für die enantiodivergente Synthese chiraler Produkte mit Aminogruppen.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201107317_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. E. Denmark, G. L. Beutner, J. Am. Chem. Soc. 2003, 125, 7800–7801.
- 2S. P. Brown, N. C. Goodwin, D. W. C. MacMillan, J. Am. Chem. Soc. 2003, 125, 1192–1194.
- 3
- 3aS. W. Smith, G. C. Fu, J. Am. Chem. Soc. 2009, 131, 14231–14232;
- 3bY. K. Chung, G. C. Fu, Angew. Chem. 2009, 121, 2259–2261;
10.1002/ange.200805377 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2225–2227;
- 3cJ. W. Sun, G. C. Fu, J. Am. Chem. Soc. 2010, 132, 4568–4569;
- 3dR. Sinisi, J. Sun, G. C. Fu, Proc. Natl. Acad. Sci. USA 2010, 107, 20652–20654.
- 4T. B. Poulsen, C. Alemparte, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 11614–11615.
- 5
- 5aS. Bertelsen, M. Marigo, S. Brandes, P. Diner, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 12973–12980;
- 5bG. Bencivenni, P. Galzerano, A. Mazzanti, G. Bartoli, P. Melchiorre, Proc. Natl. Acad. Sci. USA 2010, 107, 20642–20647;
- 5cG. Bergonzini, S. Vera, P. Melchiorre, Angew. Chem. 2010, 122, 9879–9882;
10.1002/ange.201004761 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9685–9688.
- 6
- 6aM. P. Sibi, M. Liu, Curr. Org. Chem. 2001, 5, 719–755;
- 6bG. Zanoni, F. Castronovo, M. Franzini, G. Vidari, E. Giannini, Chem. Soc. Rev. 2003, 32, 115–129;
- 6cM. Bartók, Chem. Rev. 2010, 110, 1663–1705.
- 7aY. H. Kim, Acc. Chem. Res. 2001, 34, 955–962;
- 7bJ. E. Imbriglio, M. M. Vasbinder, S. J. Miller, Org. Lett. 2003, 5, 3741–3743;
- 7cS. H. Chen, B. C. Hong, C. F. Su, S. Sarshar, Tetrahedron Lett. 2005, 46, 8899–8903;
- 7dN. Abermil, G. Masson, J. Zhu, Org. Lett. 2009, 11, 4648–4651.
- 8Y. Sohtome, S. Tanaka, K. Takada, T. Yamaguchi, K. Nagasawa, Angew. Chem. 2010, 122, 9440–9443;
10.1002/ange.201005109 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9254–9257.
- 9C. Fehr, Angew. Chem. 1996, 108, 2726–2748;
10.1002/ange.19961082204 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2566–2587.
- 10Selected recent examples:
- 10aH. Ube, N. Shimada, M. Terada, Angew. Chem. 2010, 122, 1902–1905;
10.1002/ange.200906647 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1858–1861;
- 10bT. Misaki, G. Takimoto, T. Sugimura, J. Am. Chem. Soc. 2010, 132, 6286–6287;
- 10cS. Dong, X. Liu, X. Chen, F. Mei, Y. Zhang, B. Gao, L. Lin, X. Feng, J. Am. Chem. Soc. 2010, 132, 10650–10651;
- 10dY. Sohtome, B. Shin, N. Horitsugi, R. Takagi, K. Noguchi, K. Nagasawa, Angew. Chem. 2010, 122, 7457–7461;
10.1002/ange.201003172 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7299–7303.
- 11Z. Jiang, W. Ye, Y. Zhao, S. L. M. Goh, D. Leow, Y.-T. Soh, C.-H. Tan, Adv. Synth. Catal. 2007, 349, 2454–2458.
- 12R. Yazaki, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 5522–5531.
- 13Y. Pan, Y. Zhao, T. Ma, Y. Yang, H. Liu, Z. Jiang, C.-H. Tan, Chem. Eur. J. 2010, 16, 779–782.
- 14
- 14aY. Zhao, D. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241;
- 14bA. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. B 2009, 113, 6378–6396;
- 14cGaussian 09, Revision A.02, M. J. Frisch, et al., Inc., Wallingford CT, 2009.
- 15NBO Version 3.1, E. D. Glendening, A. E. Reed, J. E. Carpenter, F. Weinhold.
- 16
- 16aZ. Jiang, Y. Pan, Y. Zhao, T. Ma, R. Lee, Y. Yang, K. W. Huang, M. Wong, C.-H. Tan, Angew. Chem. 2009, 121, 3681–3685; Angew. Chem. Int. Ed. 2009, 48, 3627–3631;
- 16bR. Lee, X. Lim, T. Chen, G. Tan, C.-H. Tan, K.-W. Huang, Tetrahedron Lett. 2009, 50, 1560–1562;
- 16cX. Fu, C.-H. Tan, Chem. Commun. 2011, 47, 8210–8222;
- 16dB. Cho, C.-H. Tan, M. W. Wong, Org. Biomol. Chem. 2011, 9, 4550–4557.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.