Efficient Heterogeneous Epoxidation of Alkenes by a Supported Tungsten Oxide Catalyst†
Dr. Keigo Kamata
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/
Search for more papers by this authorDr. Koji Yonehara
Advanced Materials Research Center, Nippon Shokubai Co., Ltd. 5-8 Nishi Otabi-cho, Suita, Osaka 564-8512 (Japan)
Search for more papers by this authorYasutaka Sumida
Advanced Materials Research Center, Nippon Shokubai Co., Ltd. 5-8 Nishi Otabi-cho, Suita, Osaka 564-8512 (Japan)
Search for more papers by this authorKazuhisa Hirata
Advanced Materials Research Center, Nippon Shokubai Co., Ltd. 5-8 Nishi Otabi-cho, Suita, Osaka 564-8512 (Japan)
Search for more papers by this authorSusumu Nojima
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/
Search for more papers by this authorCorresponding Author
Prof. Dr. Noritaka Mizuno
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/Search for more papers by this authorDr. Keigo Kamata
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/
Search for more papers by this authorDr. Koji Yonehara
Advanced Materials Research Center, Nippon Shokubai Co., Ltd. 5-8 Nishi Otabi-cho, Suita, Osaka 564-8512 (Japan)
Search for more papers by this authorYasutaka Sumida
Advanced Materials Research Center, Nippon Shokubai Co., Ltd. 5-8 Nishi Otabi-cho, Suita, Osaka 564-8512 (Japan)
Search for more papers by this authorKazuhisa Hirata
Advanced Materials Research Center, Nippon Shokubai Co., Ltd. 5-8 Nishi Otabi-cho, Suita, Osaka 564-8512 (Japan)
Search for more papers by this authorSusumu Nojima
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/
Search for more papers by this authorCorresponding Author
Prof. Dr. Noritaka Mizuno
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan) http://park.itc.u-tokyo.ac.jp/mizuno/Search for more papers by this authorThis work was supported by the Global COE Program (Chemistry Innovation through Cooperation of Science and Engineering), the Research and Development in a New Interdisciplinary Field Based on Nanotechnology and Materials Science Program, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan.
Graphical Abstract
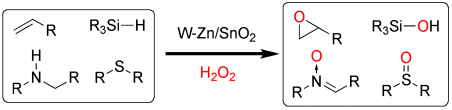
Optimierte Oxidation: Eine Kombination von Wolfram- und Zinkoxid auf einem SnO2-Träger (W–Zn/SnO2) dient als Heterogenkatalysator in der selektiven Oxidation von Alkenen, Aminen, Silanen, Sulfiden und anderen Substraten mit wässrigem H2O2 (siehe Schema). Die Produkte werden in hohen Ausbeuten erhalten, und der Katalysator kann ohne größere Leistungseinbuße mehrmals wiederverwendet werden.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201106064_sm_miscellaneous_information.pdf688.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. S. Lane, K. Burgess, Chem. Rev. 2003, 103, 2457–2474;
- 1bS. T. Oyama in Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis (Ed.: ), Elsevier, Amsterdam, 2008, pp. 1–99.
- 2J. Hagen, Industrial Catalysis: A Practical Approach, Wiley-VCH, Weinheim, 1999.
- 3
- 3aD. E. de Vos, B. F. Sels, P. A. Jacobs, Adv. Catal. 2001, 46, 1–87;
- 3bR. A. Sheldon, I. W. C. E. Arends, U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, 2007;
10.1002/9783527611003 Google Scholar
- 3c Modern Heterogeneous Oxidation Catalysis (Ed.: ), Wiley-VCH, Weinheim, 2009.
- 4
- 4aT. Okuhara, N. Mizuno, M. Misono, Adv. Catal. 1996, 41, 113–252;
- 4bM. T. Pope in Comprehensive Coordination Chemistry II, Vol. 4 (Eds.: ), Elsevier Pergamon, Amsterdam, 2004, pp. 635–678;
10.1016/B0-08-043748-6/03035-8 Google Scholar
- 4cC. L. Hill in Comprehensive Coordination Chemistry II, Vol. 4 (Eds.: ), Elsevier Pergamon, Amsterdam, 2004, pp. 679–759;
- 4dD.-L. Long, R. Tsunashima, L. Cronin, Angew. Chem. 2010, 122, 1780–1803; Angew. Chem. Int. Ed. 2010, 49, 1736–1758.
- 5
- 5aR. Neumann, M. Cohen, Angew. Chem. 1997, 109, 1810–1812; Angew. Chem. Int. Ed. Engl. 1997, 36, 1738–1740;
- 5bD. H. Koo, M. Kim, S. Chang, Org. Lett. 2005, 7, 5015–5018;
- 5cD. Hoegaerts, B. F. Sels, D. E. De Vos, F. Verpoort, P. A. Jacobs, Catal. Today 2000, 60, 209–218;
- 5dK. Yamaguchi, C. Yoshida, S. Uchida, N. Mizuno, J. Am. Chem. Soc. 2005, 127, 530–531;
- 5eN. V. Maksimchuk, K. A. Kovalenko, S. S. Arzumanov, Y. A. Chesalov, M. S. Melgunov, A. G. Stepanov, V. P. Fedin, O. A. Kholdeeva, Inorg. Chem. 2010, 49, 2920–2930.
- 6
- 6aV. Lebarbier, G. Clet, M. Houalla, J. Phys. Chem. B 2006, 110, 13905–13911;
- 6bW. Zhou, E. I. Ross-Medgaarden, W. V. Knowles, M. S. Wong, I. E. Wachs, C. J. Kiely, Nat. Chem. 2009, 1, 722–728.
- 7
- 7aS. Huang, F. Chen, S. Liu, Q. Zhu, X. Zhu, W. Xin, Z. Feng, C. Li, Q. Wang, L. Xu, J. Mol. Catal. A 2007, 267, 224–233;
- 7bD. J. Moodley, C. van Schalkwyk, A. Spamer, J. M. Botha, A. K. Datye, Appl. Catal. A 2007, 318, 155–159.
- 8
- 8aY. Li, H. Cheng, D. Li, Y. Qin, Y. Xie, S. Wang, Chem. Commun. 2008, 1470–1472;
- 8bP. Forzatti, I. Nova, E. Tronconi, Angew. Chem. 2009, 121, 8516–8518;
10.1002/ange.200903857 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8366–8368.
- 9
- 9aM. M. Ostromecki, L. J. Burcham, I. E. Wachs, N. Ramani, J. G. Ekerdt, J. Mol. Catal. A 1998, 132, 43–57;
- 9bM. M. Ostromecki, L. J. Burcham, I. E. Wachs, J. Mol. Catal. A 1998, 132, 59–71.
- 10The selectivity for 2 a with W/SnO2 was low because of the acid-catalyzed ring opening of 2 a. It has been reported that supported tungsten oxide and tin–tungsten mixed oxides act as strong solid acids:
- 10aJ. Macht, C. D. Baertsch, M. May-Lozano, S. L. Soled, Y. Wang, E. Iglesia, J. Catal. 2004, 227, 479–491;
- 10bY. Ogasawara, S. Uchida, K. Yamaguchi, N. Mizuno, Chem. Eur. J. 2009, 15, 4343–4349.
- 11R. A. Sheldon, M. Wallau, I. W. C. E. Arends, U. Schuchardt, Acc. Chem. Res. 1998, 31, 485–493.
- 12aA. Corma, M. A. Camblor, P. Esteve, A. Martínez, J. Pérez-Pariente, J. Catal. 1994, 145, 151–158;
- 12bA. Corma, M. T. Navarro, J. Pérez-Pariente, J. Chem. Soc. Chem. Commun. 1994, 147–148;
- 12cR. Hutter, D. C. M. Dutoit, T. Mallat, M. Schneider, A. Baiker, J. Chem. Soc. Chem. Commun. 1995, 163–164;
- 12dP. Wu, D. Nuntasri, J. Ruan, Y. Liu, M. He, W. Fan, O. Terasaki, T. Tatsumi, J. Phys. Chem. B 2004, 108, 19126–19131.
- 13
- 13aK. Sato, M. Aoki, T. Hashimoto, D. Panyella, R. Noyori, Bull. Chem. Soc. Jpn. 1997, 70, 905–915;
- 13bY. Ishii, K. Yamawaki, T. Ura, H. Yamada, T. Yoshida, M. Ogawa, J. Org. Chem. 1988, 53, 3581–3593;
- 13cK. Kamata, R. Ishimoto, T. Hirano, S. Kuzuya, K. Uehara, N. Mizuno, Inorg. Chem. 2010, 49, 2471–2478.
- 14Since some TONs based on W content are underestimated, the TONs based on the amounts of catalysts were compared. The TON of the W–Zn/SnO2 system was still larger than those of the other systems (56–648) under the approximately stoichiometric conditions.
- 15J. F. Scott, J. Chem. Phys. 1970, 53, 852–853.
- 16
- 16aM. A. Vuurman, I. E. Wachs, A. M. Hirt, J. Phys. Chem. 1991, 95, 9928–9937;
- 16bD. S. Kim, M. Ostromecki, I. E. Wachs, S. D. Kohler, J. G. Ekerdt, Catal. Lett. 1995, 33, 209–215;
- 16cD. Gazzoli, M. Valigi, R. Dragone, A. Marucci, G. Mattei, J. Phys. Chem. B 1997, 101, 11129–11135;
- 16dN. Vaidyanathan, D. M. Hercules, M. Houalla, Anal. Bioanal. Chem. 2002, 373, 547–554;
- 16eG. D. Panagiotou, T. Petsi, K. Bourikas, C. Kordulis, A. Lycourghiotis, J. Catal. 2009, 262, 266–279.
- 17aS. Himeno, M. Yoshihara, M. Maekawa, Inorg. Chim. Acta 2000, 298, 165–171;
- 17bB. Courcot, A. J. Bridgeman, J. Phys. Chem. A 2009, 113, 10540–10548.
- 18The XRD patterns of W–Zn/SnO2 (Zn: 0.8 wt %, W: 0.7–4.8 wt %) were the same as that of SnO2 (see Figure S3 in the Supporting Information) and showed no evidence of WO3.
- 19It has been reported that dioxomolybdenum and dioxotungsten compounds are effective catalysts for epoxidation with H2O2 and tert-butyl hydroperoxide:
- 19aF. E. Kühn, A. M. Santos, I. S. Gonçalves, C. C. Romão, A. D. Lopes, Appl. Organomet. Chem. 2001, 15, 43–50;
- 19bF. E. Kühn, A. M. Santos, W. A. Herrmann, Dalton Trans. 2005, 2483–2491;
- 19cF. E. Kühn, A. M. Santos, M. Abrantes, Chem. Rev. 2006, 106, 2455–2475.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.