Total Synthesis of Sialic Acid by a Sequential Rhodium-Catalyzed Aziridination and Barbier Allylation of D-Glycal†
Dr. Rujee Lorpitthaya
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Sharad B. Suryawanshi
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorSiming Wang
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorKalyan Kumar Pasunooti
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorShuting Cai
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Jimei Ma
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Xue-Wei Liu
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorDr. Rujee Lorpitthaya
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Sharad B. Suryawanshi
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorSiming Wang
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorKalyan Kumar Pasunooti
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorShuting Cai
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Jimei Ma
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Xue-Wei Liu
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorWe thank Prof. Francois Mathey and Prof. Koichi Narasaka for fruitful discussions. We also thank Dr. Yong Xin Li for X-ray analyses and gratefully acknowledge Nanyang Technological University (NTU; RG 50/08) and the Ministry of Health (MOH; NMRC/H1N1R/001/2009) Singapore for financial support.
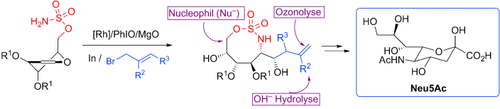
Graphical Abstract
Flexible Sequenz: Die Sialinsäure Neu5Ac wurde regio- und stereoselektiv aus einem Glycal über ein [1,2,3]-Oxathiazocan-2,2-dioxid-Intermediat synthetisiert (siehe Schema). Die Reaktionssequenz aus Rh-katalysierter Aziridinierung und Indium-vermittelter Barbier-Allylierung könnte eine flexible Route zu verschiedensten Sialinsäuren bieten, denn das Oxathiazocan war kompatibel mit einer Reihe von Nucleophilen.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201104516_sm_miscellaneous_information.pdf18.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. I. Gama, L. C. Hsieh-Wilson, Curr. Opin. Chem. Biol. 2005, 9, 609–619;
- 1bS. Mizuguchi, T. Uyama, H. Kitagawa, K. H. Nomura, K. Dejima, K. Gengyo-Ando, S. Nitani, K. Sugahara, K. Nomura, Nature 2003, 423, 443–448;
- 1cE. J. Bradbury, L. D. F. Moon, R. J. Popat, V. R. King, G. S. Bennett, P. N. Patel, J. W. Fawcett, S. B. MacMahon, Nature 2002, 416, 636–640.
- 2
- 2aL. Poletti, L. Lay, Eur. J. Org. Chem. 2003, 2999–3024;
- 2bR. J. Linhardt, T. Toida, Acc. Chem. Res. 2004, 37, 431–438.
- 3R. S. Dahl, N. S. Finney, J. Am. Chem. Soc. 2004, 126, 8356–8357.
- 4J. Du Bois, C. S. Tomooka, J. Hong, E. M. Carreira, J. Am. Chem. Soc. 1997, 119, 3179–3180.
- 5
- 5aC. Kan, C. M. Long, M. Paul, C. M. Ring, S. E. Tully, C. M. Rojas, Org. Lett. 2001, 3, 381–384;
- 5bE. Levites-Agababa, E. Menhaji, L. N. Perlson, C. M. Rojas, Org. Lett. 2002, 4, 863–865.
- 6
- 6aJ. Zeng, S. Vedachalam, S. Xiang, X.-W. Liu, Org. Lett. 2011, 13, 42–45;
- 6bF. Ding, R. William, F. Wang, J. Ma, L. Ji, X.-W. Liu, Org. Lett. 2011, 13, 652–655;
- 6cJ. Ma, Y. Zhao, S. Ng, J. Zhang, J. Zeng, A. Than, P. Chen, X.-W. Liu, Chem. Eur. J. 2010, 16, 4533–4540.
- 7
- 7aR. Lorpitthaya, Z. Z. Xie, J. L. Kuo, X.-W. Liu, Chem. Eur. J. 2008, 14, 1561–1570;
- 7bR. Lorpitthaya, K. B. Sophy, J. L. Kuo, X.-W. Liu, Org. Biomol. Chem. 2009, 7, 1284–1287;
- 7cR. Lorpitthaya, Z. Z. Xie, K. B. Sophy, J. L. Kuo, X.-W. Liu, Chem. Eur. J. 2010, 16, 588–594.
- 8For review, see C. J. Li, Chem. Rev. 2005, 105, 3095–3166.
- 9
- 9aN. Lubin-Germain, J.-P. Baltaze, A. Coste, A. Hallonet, H. Lauréano, G. Legrave, J. Uziel, J. Augé, Org. Lett. 2008, 10, 725–728;
- 9bJ. S. Yadav, B. V. S. Reddy, M. Sreenivas, G. Satheesh, Synthesis 2007, 1712–1716;
- 9cD. Mukherjee, S. K. Sarkar, U. S. Chowdhury, S. C. Taneja, Tetrahedron Lett. 2007, 48, 663–667.
- 10See the Supporting Information. An alternative mechanism is proposed to explain the regio and stereoselectivity of aziridination and indium-mediated allylation or propargylation. A DFT calculation supports the proposed mechanism.
- 11
- 11aA. Gottschalk, Nature 1951, 167, 845–847;
- 11bA. P. Corfield, R. Schauer in Sialic Acids, Chemistry, Metabolism and Function (Ed.: ), Springer, New York, 1982, pp. 5–39;
10.1007/978-3-7091-8680-0_2 Google Scholar
- 11cJ. F. G. Vliegenthart, J. P. Mamerling in Sialic Acids, Chemistry, Metabolism and Function (Ed.: ), Springer, New York, 1982, pp. 59–76;
10.1007/978-3-7091-8680-0_4 Google Scholar
- 11dR. Roy, C. A. Laferrière, R. A. Pon, Methods Enzymol. 1994, 247, 351–361.
- 12
- 12aM. von Itzstein, W. Y. Wu, G. B. Kok, M. S. Pegg, J. C. Dyason, B. Jin, P. T. Van, M. L. Smythe, H. F. White, S. W. Oliver, Nature 1993, 363, 418–423;
- 12bC. U. Kim, W. Lew, M. A. Williams, H. Liu, L. Zhang, S. Swaminathan, N. Bischofberger, M. S. Chen, D. B. Mendel, C. Y. Tai, W. G. Laver, R. C. Stevens, J. Am. Chem. Soc. 1997, 119, 681–690;
- 12cA. Moscona, N. Engl. J. Med. 2005, 353, 1363–1373.
- 13J. W. Cornforth, M. E. Firth, A. Gottschalk, J. Biol. Chem. 1958, 68, 57–61.
- 14
- 14aJ. Calveras, Y. Nagai, I. Sultana, Y. Ueda, T. Higashi, M. Shoji, T. Sugai, Tetrahedron 2010, 66, 4284–4291;
- 14bZ. Hong, L. Liu, C.-C. Hsu, C.-H. Wong, Angew. Chem. 2006, 118, 7577–7581; Angew. Chem. Int. Ed. 2006, 45, 7417–7421;
- 14cS. J. Danishefsky, M. P. DeNinno, S. H. Chen, J. Am. Chem. Soc. 1988, 110, 3929–3940;
- 14dS. J. Danishefsky, W. H. Pearson, B. E. Segmuller, J. Am. Chem. Soc. 1985, 107, 1280–1285;
- 14eS. H. Kang, H. W. Choi, J. S. Kim, J. H. Youn, Chem. Commun. 2000, 227–228;
- 14fE. A. Voight, C. Rein, S. D. Burke, J. Org. Chem. 2002, 67, 8489–8499;
- 14gA. Dondoni, A. Marra, P. Merino, J. Am. Chem. Soc. 1994, 116, 3324–3336;
- 14hA. Dondoni, A. Marra, A. Boscarato, Chem. Eur. J. 1999, 5, 3562–3572;
10.1002/(SICI)1521-3765(19991203)5:12<3562::AID-CHEM3562>3.0.CO;2-W CAS Web of Science® Google Scholar
- 14iT. Takahashi, H. Tsukamoto, M. Kurosaki, H. Yamada, Synlett 1997, 1065–1066;
- 14jM. Banwell, C. Desavi, K. Watson, J. Chem. Soc. Perkin Trans. 1 1998, 2251–2252;
- 14kL. S. Li, Y. L. Li, Y. Wu, Org. Lett. 2000, 2, 891–894;
- 14lE. A. Voight, C. R. Rein, S. D. Burke, Tetrahedron Lett. 2001, 42, 8747–8749;
- 14mK. G. Liu, S. Yan, Y. L. Wu, Z. J. Yao, J. Org. Chem. 2002, 67, 6758–6763.
- 15
- 15aW. Fitz, M. J. Kim, W. J. Hennen, H. M. Sweers, C.-H. Wong, J. Am. Chem. Soc. 1988, 110, 6481–6486;
- 15bC. Auge, S. David, C. Gautheron, Tetrahedron Lett. 1984, 25, 4663–4664;
- 15cT. H. Chan, M. C. Lee, J. Org. Chem. 1995, 60, 4228–4232;
- 15dD. M. Gordon, G. M. Whitesides, J. Org. Chem. 1993, 58, 7937–7938;
- 15eM. Warwel, W. Fessner, Synlett 2000, 865–867;
- 15fT. H. Chan, C. J. Li, J. Chem. Soc. Chem. Commun. 1992, 747–748.
- 16
- 16aB. Aguilera, A. Fernandez-Mayoralas, J. Org. Chem. 1998, 63, 2719–2723;
- 16bH. M. Chen, S. G. Withers, Carbohydr. Res. 2007, 342, 2212–2222;
- 16cC. G. Espino, P. M. Wehn, J. Chow, J. Du Bois, J. Am. Chem. Soc. 2001, 123, 6935–6936;
- 16dS. Toumieux, P. Compain, O. R. Martin, J. Org. Chem. 2008, 73, 2155–2162.
- 17CCDC 846689 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.