Experimental Diels–Alder Reactivities of Cycloalkenones and Cyclic Dienes Explained through Transition-State Distortion Energies†
We are grateful to the John Fell Oxford University Press Research Fund (R.S.P) and the National Science Foundation (CHE-0548209 and Graduate Fellowship to A.G.R.) for financial support of this research. Computer time was provided in part by the UCLA Institute for Digital Research and Education (IDRE), by the Shared Research Computing Services Pilot (ShaRCS) project for the University of California Systems, and by the National Center for Supercomputing Applications on Cobalt, TG-CHE050044N, and Abe, TG-CHE090070.
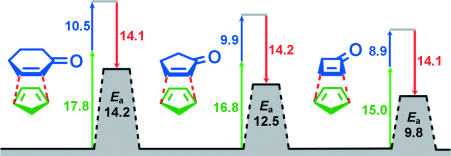
Graphical Abstract
Quantenchemische Rechnungen werden zur Untersuchung experimentell gemessener Reaktivitäten von cyclischen Dienen und Cycloalkenonen in Diels-Alder-Reaktionen verwendet. Die Wechselwirkungsenergien (rot) sind nahezu konstant; Unterschiede ergeben sich durch verschiedene Verzerrungsenergien von Dienophilen (blau) und Dienen (grün; siehe Bild, Ea=Aktivierungsenergie; Angaben in kcal mol−1).