Amino Acid-Based Reoxidants for Aminohydroxylation: Application to the Construction of Amino Acid–Amino Alcohol Conjugates†
Corresponding Author
Prof. Timothy J. Donohoe
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)Search for more papers by this authorDr. Cedric K. A. Callens
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDr. Aida Flores
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDr. Stefanie Mesch
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDarren L. Poole
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorIshmael A. Roslan
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorCorresponding Author
Prof. Timothy J. Donohoe
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)Search for more papers by this authorDr. Cedric K. A. Callens
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDr. Aida Flores
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDr. Stefanie Mesch
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDarren L. Poole
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorIshmael A. Roslan
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorWe thank the EPSRC, the European Union, the SNSF, and AstraZeneca for supporting this project. We also thank the Oxford Chemical Crystallography Service for the use of their instrumentation.
Graphical Abstract
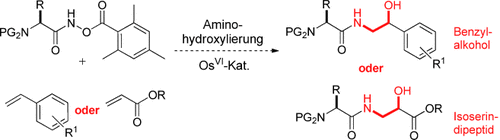
Eine praktische Stickstoffquelle für die Aminohydroxylierung terminaler Alkene: Die Zugabe eines N-O-basierten Reoxidationsmittel zu einem Aminosäure-Acylkohlenstoffatom ergab Verbindungen, die den katalytischen Umsatz erleichterten und die Konjugation von Aminosäure und Alken vermittelten. Die Regioselektivität war hoch, und die durch katalytische Mengen eines chiralen Liganden erreichte Stereoselektivität war ebenfalls gut.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201103293_sm_miscellaneous_information.pdf3.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. J. Donohoe, C. K. A. Callens, A. Flores, A. R. Lacy, A. H. Rath, Chem. Eur. J. 2011, 17, 58;
- 1bE. J. Alexanian, C. Lee, E. J. Sorensen, J. Am. Chem. Soc. 2005, 127, 7690;
- 1cD. J. Wardrop, E. G. Bowen, R. E. Forslund, A. D. Sussman, S. L. Weerasekera, J. Am. Chem. Soc. 2010, 132, 1188;
- 1dK. S. Williamson, T. P. Yoon, J. Am. Chem. Soc. 2010, 132, 4570;
- 1eT. de Haro, C. Nevado, Angew. Chem. 2011, 123, 936;
10.1002/ange.201005763 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 906.
- 2
- 2aM. Y. Kozutsumi, Y. S. Nakamura, T. Fujita, T. Kawasaki, Biochem. Biophys. Res. Commun. 1995, 211, 396;
- 2bJ. C. Madureira de Freitas, Jr., B. Du Rocher D’Aguiar Silva, F. Waldemir, M. Wallace, E. Saul Furquim, A. Werneck, J. A. Morgado-Díaz, Cancer Chemother. Pharmacol. 2011, in press;
- 2cI. Paterson, G. J. Naylor, T. Fujita, E. Guzmán, A. E. Wright, Chem. Commun. 2010, 46, 261;
- 2dS. Yamashita, Exp. Oncol. 2011, 33, 24.
- 3
- 3aH. C. Kolb, K. B. Sharpless, Transition Met. Org. Synth. 1998, 2, 243–260;
- 3bD. Nilov, O. Reiser, Adv. Synth. Catal. 2002, 344, 1169;
- 3cP. O’Brien, Angew. Chem. 1999, 111, 339;
10.1002/(SICI)1521-3757(19990201)111:3<339::AID-ANGE339>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 1999, 38, 326;10.1002/(SICI)1521-3773(19990201)38:3<326::AID-ANIE326>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 3dM. Bruncko, G. Schlingloff, K. B. Sharpless, Angew. Chem. 1997, 109, 1580;
10.1002/ange.19971091340 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1483;
- 3eZ. P. Demko, M. Bartsch, K. B. Sharpless, Org. Lett. 2000, 2, 2221.
- 4
- 4aT. J. Donohoe, M. J. Chughtai, D. J. Klauber, D. Griffin, A. D. Campbell, J. Am. Chem. Soc. 2006, 128, 2514;
- 4bT. J. Donohoe, C. J. R. Bataille, W. Gattrell, J. Kloesges, E. Rossignol, Org. Lett. 2007, 9, 1725;
- 4cT. J. Donohoe, C. K. A. Callens, A. L. Thompson, Org. Lett. 2009, 11, 2305;
- 4dL. Harris, S. P. H. Mee, R. H. Furneaux, G. J. Gainsford, A. Luxenburger, J. Org. Chem. 2011, 76, 358.
- 5
- 5aR. R. Ruffolo, J. M. Stadel, J. P. Hieble, Med. Res. Rev. 1994, 14, 229;
- 5bC. Bombelli, L. Giansanti, P. Luciani, G. Mancini, Curr. Med. Chem. 2009, 16, 171;
- 5cW. M. Pardridge, Mol. Intervention 2003, 3, 90;
- 5dF. Pinne, I. Cacciatore, C. Cornacchia, A. Mollica, P. Sozio, L. S. Cerasa, A. Iannitelli, A. Fontana, C. Nasuti, A. Di Stefano, Amino Acids 2011, DOI: .
- 6
- 6aA. G. Griesbeck, H. Mauder, Angew. Chem. 1992, 104, 97; Angew. Chem. Int. Ed. Engl. 1992, 31, 73;
- 6bA. G. Griesbeck, H. Mauder, I. Müller, Chem. Ber. 1992, 125, 2467.
- 7This compound was synthesised from commercially available N-Boc-hydroxylamine. Acylation and subsequent Boc deprotection in acidic medium afforded the O-mesitoyl hydroxylamine salt in 92 % yield over the two steps.
- 8DMTMM is 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride:
- 8aZ. J. Kamiński, P. Paneth, J. Rudziński, J. Org. Chem. 1998, 63, 4248;
- 8bA. Falchi, G. Giacomelli, A. Porcheddu, M. Taddei, Synlett 2000, 275.
- 9CCDC 824647 (7 d), 824648 (9), 824649 (11), and 824650 (16 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 10
- 10aK. L. Reddy, K. R. Dress, K. B. Sharpless, Tetrahedron Lett. 1998, 39, 3667;
- 10bP. O’Brien, S. A. Osborne, D. D. Parker, Tetrahedron Lett. 1998, 39, 4099;
- 10cD. J. Michaelis, C. J. Shaffer, T. P. Yoon, J. Am. Chem. Soc. 2007, 129, 1866; however, see:
- 10dV. Nesterenko, J. T. Byers, P. J. Hergenrother, Org. Lett. 2003, 5, 281;
- 10eP. Wu, R. Higraf, V. V. Fokin, Adv. Synth. Catal. 2006, 348, 1079.
- 11K. L. Reddy, K. B. Sharpless, J. Am. Chem. Soc. 1998, 120, 1207.
- 12
- 12aH. R. Ing, R. H. F. Manske, J. Chem. Soc. 1926, 129, 2348;
- 12bR. T. Skerlj, G. J. Bridger, A. Kaller, E. J. McEachern, J. B. Crawford, Y. Zhou, B. Atsma, J. Langille, S. Nan, D. Veale, T. Wilson, C. Harwig, S. Hatse, K. Princen, E. De Clercq, D. Schols, J. Med. Chem. 2010, 53, 3376.
- 13While the aminohydroxylation of α-methylstyrene is known (
- 13aK. B. Sharpless, D. W. Patrick, L. K. Truesdale, S. A. Biller, J. Am. Chem. Soc. 1975, 97, 2305;
- 13bK. Muñiz, I. Almodovar, J. Streuff, M. Nieger, Adv. Synth. Catal. 2006, 348, 1831), the asymmetric variant is not. Therefore, our assignment is by analogy with the asymmetric dihydroxylation reaction of α-methylstryrene, which gives the same sense of enantioselectivity as for styrene ( K. B. Sharpless, W. Amberg, Y. L. Bennani, G. A. Crispino, J. Hartung, K. S. Jeong, H. L. Kwong, K. Morikawa, Z. M. Wang, D. Xu, X. L. Zhang, J. Org. Chem. 1992, 57, 2768).
- 14The sense of stereoselectivity was assigned by analogy to related reactions, see:
- 14aG. Li, H. T. Chang, K. B. Sharpless, Angew. Chem. 1996, 108, 449; Angew. Chem. Int. Ed. Engl. 1996, 35, 451;
- 14bK. Lowpetch, D. W. Young, Org. Biomol. Chem. 2005, 3, 3348.
- 15For examples of both natural and nonnatural peptide sequences that contain isoserine and also exhibit biological activity, see:
- 15aR. Mariani, G. Granata, S. I. Maffioli, S. Serina, C. Brunati, M. Sosio, A. Marazzi, A. Vannini, D. Patel, R. White, R. Ciabatti, Bioorg. Med. Chem. Lett. 2005, 15, 3748;
- 15bB. S. Coller, K. T. Springer, L. E. Scudder, J. L. Kutok, M. Ceruso, G. D. Prestwich, J. Biol. Chem. 1993, 268, 20741.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.