Hydrogen Storage in Magnesium Hydride: The Molecular Approach†
Corresponding Author
Prof. Dr. Sjoerd Harder
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen (Netherlands), Fax: (+31) 50-363-4296
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen (Netherlands), Fax: (+31) 50-363-4296Search for more papers by this authorDr. Jan Spielmann
Fakultät für Chemie, Universität Duisburg-Essen, Universitätsstrasse 5, 45117 Duisburg (Germany)
Search for more papers by this authorJulia Intemann
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen (Netherlands), Fax: (+31) 50-363-4296
Search for more papers by this authorHeinz Bandmann
Fakultät für Chemie, Universität Duisburg-Essen, Universitätsstrasse 5, 45117 Duisburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Sjoerd Harder
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen (Netherlands), Fax: (+31) 50-363-4296
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen (Netherlands), Fax: (+31) 50-363-4296Search for more papers by this authorDr. Jan Spielmann
Fakultät für Chemie, Universität Duisburg-Essen, Universitätsstrasse 5, 45117 Duisburg (Germany)
Search for more papers by this authorJulia Intemann
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen (Netherlands), Fax: (+31) 50-363-4296
Search for more papers by this authorHeinz Bandmann
Fakultät für Chemie, Universität Duisburg-Essen, Universitätsstrasse 5, 45117 Duisburg (Germany)
Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft for financial support of this project. Prof. Dr. R. Boese and D. Bläser are kindly acknowledged for collection of X-ray data.
Graphical Abstract
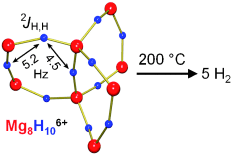
Groß und klein zugleich: Der größte ligandenstabilisierte Magnesiumhydridcluster, bestehend aus 8 Mg2+- und 10 H−-Ionen, dient als Modell für das kleinste Wasserstoffspeichermaterial im Subnanometerbereich. Zwischen Hydrid-Ionen in diesem molekularen Cluster werden magnetische Kopplungen beobachtet (siehe Bild), und ein Erwärmen auf lediglich 200 °C genügt, um den Wasserstoff vollständig zu desorbieren.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201101153_sm_miscellaneous_information.pdf157.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Schlapbach, A. Züttel, Nature 2001, 414, 353–358.
- 2For recent reviews, see:
- 2aT. B. Marder, Angew. Chem. 2007, 119, 8262–8264;
10.1002/ange.200703150 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8116–8118;
- 2bF. H. Stephens, V. Pons, R. T. Baker, Dalton Trans. 2007, 2613–2626;
- 2cT. J. Clark, K. Lee, I. Manners, Chem. Eur. J. 2006, 12, 8634–8648;
- 2dA. Karkambar, C. Aardahl, T. Autrey, Mater. Matters 2007, 2, 6–10;
- 2eN. C. Smythe, J. C. Gordon, Eur. J. Inorg. Chem. 2010, 509–521.
- 3
- 3aM. Dornheim, S. Doppiu, G. Barkhordarian, U. Boesenberg, T. Klassen, O. Gutfleisch, R. Bormann, Scr. Mater. 2007, 56, 841–846;
- 3bK.-F. Aguey-Zinsou, J.-R. Ares-Fernández, Energy Environ. Sci. 2010, 3, 526–543;
- 3cI. P. Jain, C. Lal, A. Jain, Int. J. Hydrogen Energy 2010, 35, 5133–5144;
- 3dC. Wu, H.-M. Cheng, J. Mater. Chem. 2010, 20, 5390–5400.
- 4B. Bogdanović, B. Spliethoff, Int. J. Hydrogen Energy 1987, 12, 863–873.
- 5P. C. M. M. Magusin, W. P. Kalisvaart, P. H. L. Notten, R. A. van Santen, Chem. Phys. Lett. 2008, 456, 55–58.
- 6P. Larsson, C. M. Araújo, J. A. Larsson, P. Jena, R. Ahuja, Proc. Natl. Acad. Sci. USA 2008, 105, 8227–8231.
- 7J. Huot, G. Liang, R. Schulz, J. Alloys Compd. 2003, 353, L12L15.
- 8W. Oelerich, T. Klassen, R. Bormann, J. Alloys Compd. 2001, 315, 237–242.
- 9X. L. Wang, S. Suda, J. Alloys Compd. 1995, 231, 380–386.
- 10J. F. Stampfer, Jr., C. E. Holley, Jr., J. F. Suttle, J. Am. Chem. Soc. 1960, 82, 3504–3508.
- 11C. M. Stander, R. A. Pacey, J. Phys. Chem. Solids 1978, 39, 829–832.
- 12R. W. P. Wagemans, J. H. van Lenthe, P. E. de Jongh, A. J. van Dillen, K. P. de Jong, J. Am. Chem. Soc. 2005, 127, 16675–16680.
- 13P. E. de Jongh, R. W. P. Wagemans, T. T. M. Eggenhuisen, B. S. Dauvillier, P. B. Radstake, J. D. Meeldijk, J. W. Geus, K. P. de Jong, Chem. Mater. 2007, 19, 6052–6057.
- 14L. Haas, A. Gedanken, Chem. Commun. 2008, 1795–1797.
- 15S. Zhang, A. F. Gross, S. L. Van Atta, L. Maribel, P. Liu, C. C. Ahn, J. J. Vajo, C. M. Jensen, Nanotechnology 2009, 20, 204027.
- 16M. Paskevicius, D. A. Sheppard, C. E. Buckley, J. Am. Chem. Soc. 2010, 132, 5077–5083.
- 17Z. Zhao-Karger, J. Hu, A. Roth, D. Wang, C. Kübel, W. Lohstroh, M. Fichtner, Chem. Commun. 2010, 46, 8353–8355.
- 18Lowering of the decomposition temperature is somewhat less than expected: the concomitant decrease in ΔS partially counteracts the lower ΔH value, see Refs. [16, 17].
- 19S. Harder, J. Brettar, Angew. Chem. 2006, 118, 3554–3558; Angew. Chem. Int. Ed. 2006, 45, 3474–3478.
- 20
- 20aS. P. Green, C. Jones, A. Stasch, Angew. Chem. 2008, 120, 9219–9223; Angew. Chem. Int. Ed. 2008, 47, 9079–9083;
- 20bS. J. Bonyhady, C. Jones, S. Nembenna, A. Stasch, A. J. Edwards, G. J. McIntyre, Chem. Eur. J. 2010, 16, 938–955.
- 21S. P. Green, C. Jones, A. Stasch, Science 2007, 318, 1754–1757.
- 22S. J. Bonyhady, D. Collis, G. Frenking, N. Holzmann, C. Jones, A. Stasch, Nat. Chem. 2010, 2, 865–869.
- 23M. J. Michalczyk, Organometallics 1992, 11, 2307.
- 24
- 24aF. Buch, J. Brettar, S. Harder, Angew. Chem. 2006, 118, 2807–2811;
10.1002/ange.200504164 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2741–2745;
- 24bS. Harder, Chem. Rev. 2010, 110, 3852–3876.
- 25M. Arrowsmith, M. S. Hill, D. J. MacDougall, M. F. Mahon, Angew. Chem. 2009, 121, 4073–4076;
10.1002/ange.200900878 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4013–4016.
- 26
- 26aC. Ruspic, S. Nembenna, A. Hofmeister, J. Magull, S. Harder, H. W. Roesky, J. Am. Chem. Soc. 2006, 128, 15000–15004;
- 26bS. Nembenna, H. W. Roesky, S. Nagendran, A. Hofmeister, J. Magull, P.-J. Wilbrandt, M. Hahn, Angew. Chem. 2007, 119, 2564–2566;
10.1002/ange.200604447 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2512–2514;
- 26cC. Ruspic, S. Harder, Inorg. Chem. 2007, 46, 10427–10433.
- 27D. F.-J. Piesik, S. Range, S. Harder, Organometallics 2008, 27, 6178–6187;
- 27bD. F.-J. Piesik, R. Stadler, S. Range, S. Harder, Eur. J. Inorg. Chem. 2009, 3569–3576.
- 28[paraMg] could not be detected in the NMR spectrum of the reaction mixture.
- 29
- 29aR. E. Mulvey, Chem. Commun. 2001, 1049–1056;
- 29bD. J. Gallagher, K. W. Henderson, A. R. Kennedy, C. T. O’Hara, R. E. Mulvey, R. B. Rowlings, Chem. Commun. 2002, 376–377.
- 30J. A. Pople, B. T. Luke, M. J. Frisch, J. S. Binkley, J. Chem. Phys. 1985, 83, 2198–2203.
- 31W. H. Zachariasen, C. E. Holley, Jr., J. F. Stamper, Jr., Acta Crystallogr. 1963, 16, 352–353.
- 32S. Hayashi, J. Alloys Compd. 1997, 248, 66–69.
- 33I. Alkorta, P. F. Provasi, G. A. Aucar, J. Elguero, Magn. Reson. Chem. 2008, 46, 356–361.
- 34T. C. Farrar, G. R. Quinting, Inorg. Chem. 1985, 24, 1941–1945.
- 35S. Aldridge, A. J. Downs, Chem. Rev. 2001, 101, 3305–3365.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.