Amplification of Chirality by Supramolecular Polymerization of Pyrene Oligomers†
Alina L. Nussbaumer
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057
Search for more papers by this authorDaniel Studer
Institute of Anatomy, University of Bern, Baltzerstrasse 2, 3000 Bern 9 (Switzerland)
Search for more papers by this authorVladimir L. Malinovskii
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057
Search for more papers by this authorCorresponding Author
Robert Häner
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057Search for more papers by this authorAlina L. Nussbaumer
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057
Search for more papers by this authorDaniel Studer
Institute of Anatomy, University of Bern, Baltzerstrasse 2, 3000 Bern 9 (Switzerland)
Search for more papers by this authorVladimir L. Malinovskii
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057
Search for more papers by this authorCorresponding Author
Robert Häner
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern (Switzerland), Fax: (+41) 31-631-8057Search for more papers by this authorFinancial support by the Swiss National Foundation (grant 200020-132581) is gratefully acknowledged. The authors thank Prof. E. Castiglioni (Dipartimento di Scienze Biomediche e Biotecnologie, Università di Brescia, 25123 Brescia, Italy) for help with LD experiments.
Graphical Abstract
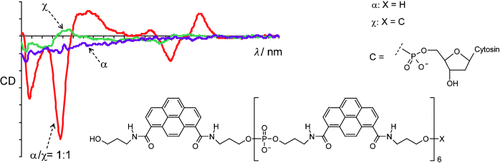
Achirale Pyrenoligomere α zeigen eine bemerkenswerte Chiralitätsverstärkung (siehe Circulardichroismusspektren) in Gegenwart geringster Mengen eines chiralen Induktors χ. Spektroskopische und elektronenmikroskopische Daten stützen ein Modell, dem zufolge die Pyrenoligomere durch Stapelwechselwirkungen helicale supramolekulare Polymere bilden.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201100677_sm_miscellaneous_information.pdf5.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Brunsveld, B. J. B. Folmer, E. W. Meijer, R. P. Sijbesma, Chem. Rev. 2001, 101, 4071–4097.
- 2J. M. Lehn, Polym. Int. 2002, 51, 825–839.
- 3S. Mann, Nat. Mater. 2009, 8, 781–792.
- 4J. M. Lehn, Supramolecular Chemistry—Concepts and Perspectives, VCH, Weinheim, 1995.
10.1002/3527607439 Google Scholar
- 5G. M. Whitesides, J. P. Mathias, C. T. Seto, Science 1991, 254, 1312–1319.
- 6J. S. Lindsey, New J. Chem. 1991, 15, 153–180.
- 7V. Percec, M. Glodde, T. K. Bera, Y. Miura, I. Shiyanovskaya, K. D. Singer, V. S. K. Balagurusamy, P. A. Heiney, I. Schnell, A. Rapp, H. W. Spiess, S. D. Hudson, H. Duan, Nature 2002, 417, 384–387.
- 8H. J. Schneider, Angew. Chem. 2009, 121, 3982–4036;
10.1002/ange.200802947 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3924–3977.
- 9D. Pijper, B. L. Feringa, Soft Matter 2008, 4, 1349–1372.
- 10A. W. Bosman, R. P. Sijbesma, E. W. Meijer, Mater. Today 2004, 7, 34–39.
- 11J. D. Badjic, A. Nelson, S. J. Cantrill, W. B. Turnbull, J. F. Stoddart, Acc. Chem. Res. 2005, 38, 723–732.
- 12J. E. Anthony, Chem. Rev. 2006, 106, 5028–5048.
- 13E. R. Kay, D. A. Leigh, F. Zerbetto, Angew. Chem. 2007, 119, 72–196; Angew. Chem. Int. Ed. 2007, 46, 72–191.
- 14L. C. Palmer, S. I. Stupp, Acc. Chem. Res. 2008, 41, 1674–1684.
- 15T. Rehm, C. Schmuck, Chem. Commun. 2008, 801–813.
- 16M. R. Wasielewski, Acc. Chem. Res. 2009, 42, 1910–1921.
- 17A. R. A. Palmans, J. A. J. M. Vekemans, E. E. Havinga, E. W. Meijer, Angew. Chem. 1997, 109, 2763–2765;
10.1002/ange.19971092328 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2648–2651.
- 18F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer, A. P. H. J. Schenning, Chem. Rev. 2005, 105, 1491–1546.
- 19T. F. A. de Greef, E. W. Meijer, Nature 2008, 453, 171–173.
- 20A. Lohr, F. Wurthner, Angew. Chem. 2008, 120, 1252–1256;
10.1002/ange.200704550 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1232–1236.
- 21X. L. Feng, W. Pisula, L. J. Zhi, M. Takase, K. Mullen, Angew. Chem. 2008, 120, 1727–1730; Angew. Chem. Int. Ed. 2008, 47, 1703–1706.
- 22V. L. Malinovskii, D. Wenger, R. Häner, Chem. Soc. Rev. 2010, 39, 410–422.
- 23J. D. Hartgerink, E. R. Zubarev, S. I. Stupp, Curr. Opin. Solid State Mater. Sci. 2001, 5, 355–361.
- 24E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. 2003, 115, 1244–1287; Angew. Chem. Int. Ed. 2003, 42, 1210–1250.
- 25K. C. Hannah, B. A. Armitage, Acc. Chem. Res. 2004, 37, 845–853.
- 26P. G. A. Janssen, A. Ruiz-Carretero, D. Gonzalez-Rodriguez, E. W. Meijer, A. P. H. J. Schenning, Angew. Chem. 2009, 121, 8247–8250;
10.1002/ange.200903507 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8103–8106.
- 27P. G. A. Janssen, S. Jabbari-Farouji, M. Surin, X. Vila, J. C. Gielen, T. F. A. de Greef, M. R. J. Vos, P. H. H. Bomans, N. A. J. M. Sommerdijk, P. C. M. Christianen, P. Leclere, R. Lazzaroni, P. van der Schoot, E. W. Meijer, A. P. H. J. Schenning, J. Am. Chem. Soc. 2009, 131, 1222–1231.
- 28R. Iwaura, M. Ohnishi-Kameyama, T. Iizawa, Chem. Eur. J. 2009, 15, 3729–3735.
- 29H. Kitagishi, K. Oohora, T. Hayashi, Biopolymers 2009, 91, 194–200.
- 30S. H. Gellman, Acc. Chem. Res. 1998, 31, 173–180.
- 31D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes, J. S. Moore, Chem. Rev. 2001, 101, 3893–4011.
- 32I. Huc, Eur. J. Org. Chem. 2004, 17–29.
- 33E. Yashima, K. Maeda in Foldamers—Structure, Properties, and Applications (Eds.: ), Wiley-VCH, Weinheim, 2007, pp. 331–366.
- 34Y. Wang, F. Li, Y. M. Han, F. Y. Wang, H. Jiang, Chem. Eur. J. 2009, 15, 9424–9433.
- 35D. Haldar, C. Schmuck, Chem. Soc. Rev. 2009, 38, 363–371.
- 36S. M. Langenegger, R. Häner, Chem. Commun. 2004, 2792–2793.
- 37S. M. Langenegger, R. Häner, ChemBioChem 2005, 6, 848–851.
- 38S. M. Langenegger, R. Häner, Bioorg. Med. Chem. Lett. 2006, 16, 5062–5065.
- 39I. Trkulja, R. Häner, Bioconjugate Chem. 2007, 18, 289–292.
- 40I. Trkulja, R. Häner, J. Am. Chem. Soc. 2007, 129, 7982–7989.
- 41F. Samain, V. L. Malinovskii, S. M. Langenegger, R. Häner, Bioorg. Med. Chem. 2008, 16, 27–33.
- 42M. H. Caruthers, Science 1985, 230, 281–285.
- 43V. L. Malinovskii, F. Samain, R. Häner, Angew. Chem. 2007, 119, 4548–4551;
10.1002/ange.200700891 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4464–4467.
- 44R. Häner, F. Samain, V. L. Malinovskii, Chem. Eur. J. 2009, 15, 5701–5708.
- 45V. A. Galievsky, V. L. Malinovskii, A. S. Stasheuski, F. Samain, K. A. Zachariasse, R. Häner, V. S. Chirvony, Photochem. Photobiol. Sci. 2009, 8, 1448–1454.
- 46R. Häner, F. Garo, D. Wenger, V. L. Malinovskii, J. Am. Chem. Soc. 2010, 132, 7466–7471.
- 47F. M. Winnik, Chem. Rev. 1993, 93, 587–614.
- 48K. A. Zachariasse, W. Kuhnle, A. Weller, Chem. Phys. Lett. 1978, 59, 375–380.
- 49K. Zachariasse, W. Kuhnle, Z. Phys. Chem. 1976, 101, 267–276.
- 50S. Werder, V. L. Malinovskii, R. Häner, Org. Lett. 2008, 10, 2011–2014.
- 51H. Bittermann, D. Siegemund, V. L. Malinovskii, R. Häner, J. Am. Chem. Soc. 2008, 130, 15285–15287.
- 52C. A. Hunter, K. R. Lawson, J. Perkins, C. J. Urch, J. Chem. Soc. Perkin Trans. 2 2001, 651–669.
- 53M. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook, S. Lifson, Science 1995, 268, 1860–1866.
- 54M. M. Green, J. W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger, J. V. Selinger, Angew. Chem. 1999, 111, 3328–3345;
10.1002/(SICI)1521-3757(19991102)111:21<3328::AID-ANGE3328>3.0.CO;2-Z Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3138–3154.10.1002/(SICI)1521-3773(19991102)38:21<3138::AID-ANIE3138>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 55A. R. A. Palmans, E. W. Meijer, Angew. Chem. 2007, 119, 9106–9126;
10.1002/ange.200701285 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8948–8968.
- 56K. Maeda, E. Yashima, Top. Curr. Chem. 2006, 265, 47–88.
- 57T. E. Kaiser, V. Stepanenko, F. Würthner, J. Am. Chem. Soc. 2009, 131, 6719–6732.
- 58P. Rivera-Fuentes, J. L. Alonso-Gomez, A. G. Petrovic, F. Santoro, N. Harada, N. Berova, F. Diederich, Angew. Chem. 2010, 122, 2296–2300;
10.1002/ange.200906191 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2247–2250.
- 59T. F. A. de Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma, E. W. Meijer, Chem. Rev. 2009, 109, 5687–5754.
- 60M. M. Green, K. S. Cheon, S. Y. Yang, J. W. Park, S. Swansburg, W. H. Liu, Acc. Chem. Res. 2001, 34, 672–680.
- 61M. M. Green, M. P. Reidy, R. J. Johnson, G. Darling, D. J. Oleary, G. Willson, J. Am. Chem. Soc. 1989, 111, 6452–6454.
- 62E. Yashima, K. Maeda, H. Iida, Y. Furusho, K. Nagai, Chem. Rev. 2009, 109, 6102–6211.
- 63F. Totsingan, V. Jain, W. C. Bracken, A. Faccini, T. Tedeschi, R. Marchelli, R. Corradini, N. R. Kallenbach, M. M. Green, Macromolecules 2010, 43, 2692–2703.
- 64N. Berova, K. Nakanishi, R. W. Woody, Circular Dichroism—Principles and Applications, 2nd ed., Wiley-VCH, New York, 2000.
- 65N. Berova, L. Di Bari, G. Pescitelli, Chem. Soc. Rev. 2007, 36, 914–931.
- 66G. A. Hembury, V. V. Borovkov, Y. Inoue, Chem. Rev. 2008, 108, 1–73.
- 67M. M. J. Smulders, A. P. H. J. Schenning, E. W. Meijer, J. Am. Chem. Soc. 2008, 130, 606–611.
- 68T. Ishi-i, R. Kuwahara, A. Takata, Y. Jeong, K. Sakurai, S. Mataka, Chem. Eur. J. 2006, 12, 763–776.
- 69M. M. J. Smulders, M. M. L. Nieuwenhuizen, T. F. A. de Greef, P. van der Schoot, A. P. H. J. Schenning, E. W. Meijer, Chem. Eur. J. 2010, 16, 362–367.
- 70M. M. Green in Circular Dichroism—Principles and Applications (Eds.: ), Wiley-VCH, Hoboken, NJ, 2000, pp. 491–520.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.