Development of a Selective, Sensitive, and Reversible Biosensor by the Genetic Incorporation of a Metal-Binding Site into Green Fluorescent Protein†
Dr. Niraikulam Ayyadurai
School of Biotechnology, Yeungnam University (South Korea)
These authors contributed equally.
Search for more papers by this authorNadarajan Saravanan Prabhu
School of Biotechnology, Yeungnam University (South Korea)
These authors contributed equally.
Search for more papers by this authorKanagavel Deepankumar
School of Biotechnology, Yeungnam University (South Korea)
Search for more papers by this authorProf. Sun-Gu Lee
Department of Chemical Engineering, Pusan National University (South Korea)
Search for more papers by this authorHeon-Ho Jeong
Department of Chemical Engineering, Chungnam National University (South Korea)
Search for more papers by this authorProf. Chang-Soo Lee
Department of Chemical Engineering, Chungnam National University (South Korea)
Search for more papers by this authorCorresponding Author
Prof. Hyungdon Yun
School of Biotechnology, Yeungnam University (South Korea)
School of Biotechnology, Yeungnam University (South Korea)Search for more papers by this authorDr. Niraikulam Ayyadurai
School of Biotechnology, Yeungnam University (South Korea)
These authors contributed equally.
Search for more papers by this authorNadarajan Saravanan Prabhu
School of Biotechnology, Yeungnam University (South Korea)
These authors contributed equally.
Search for more papers by this authorKanagavel Deepankumar
School of Biotechnology, Yeungnam University (South Korea)
Search for more papers by this authorProf. Sun-Gu Lee
Department of Chemical Engineering, Pusan National University (South Korea)
Search for more papers by this authorHeon-Ho Jeong
Department of Chemical Engineering, Chungnam National University (South Korea)
Search for more papers by this authorProf. Chang-Soo Lee
Department of Chemical Engineering, Chungnam National University (South Korea)
Search for more papers by this authorCorresponding Author
Prof. Hyungdon Yun
School of Biotechnology, Yeungnam University (South Korea)
School of Biotechnology, Yeungnam University (South Korea)Search for more papers by this authorWe thank Prof. Peter G. Schultz for his generous gift of a sample of the orthogonal tRNA/synthetase pair. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0006343).
Graphical Abstract
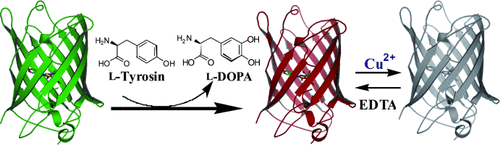
Wenn das Licht ausgeht: Ein Kupfer- Biosensor wurde durch gentechnischen Ersatz von L-Tyrosin in grün fluoreszierendem Protein durch die chelatisierende nichtkanonische Aminosäure L-DOPA hergestellt (siehe Bild). Die spezifische Bindung von Cu2+ durch das modifizierte Protein war reversibel und führte zu einer mit dem Cu2+-Gehalt skalierenden Fluoreszenzlöschung. EDTA=Ethylendiamintetraessigsäure.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201008289_sm_miscellaneous_information.pdf212.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. E. Kim, T. Nevitt, D. J. Thiele, Nat. Chem. Biol. 2008, 4, 176.
- 2
- 2aS. V. Wegner, H. Arslan, M. Sunbul, J. Yin, C. He, J. Am. Chem. Soc. 2010, 132, 2567;
- 2bS. Krupanidhi, A. Sreekumar, C. B. Sanjeevi, Indian J. Med. Res. 2008, 128, 448.
- 3Y. Rahimi, S. Shrestha, T. Banerjee, S. K. Deo, Anal. Biochem. 2007, 370, 60.
- 4
- 4aL. Jorhem, J. Engman, J. AOAC Int. 2000, 83, 1189;
- 4bY. N. Jiang, H. Q. Luo, N. B. Li, Anal. Sci. 2006, 22, 1079;
- 4cH. H. Zeng, R. B. Thompson, B. P. Maliwal, G. R. Fones, J. W. Moffett, C. A. Fierke, Anal. Chem. 2003, 75, 6807.
- 5C. Isarankura-Na-Ayudhya, T. Tantimongcolwat, H. Galla, V. Prachayasittikul, Biol. Trace Elem. Res. 2010, 134, 352.
- 6
- 6aT. A. Richmond, T. T. Takahashi, R. Shimkhada, J. Bernsdorf, Biochem. Biophys. Res. Commun. 2000, 268, 462;
- 6bN. Tansila, K. Becker, C. Isarankura Na-Ayudhya, V. Prachayasittikul, L. B. Low, Biotechnol. Lett. 2008, 30, 1391;
- 6cV. Prachayasittikul, C. Isarankura-Na-Ayudhya, H. J. Galla, Eur. Biophys. J. 2004, 33, 522;
- 6dN. Tansila, T. Tantimongcolwat, C. Isarankura-Na-Ayudhya, C. Nantasenamat, V. Prachayasittikul, Int. J. Biol. Sci. 2007, 3, 463.
- 7S. Shrestha, S. K. Deo, Anal. Bioanal. Chem. 2006, 386, 515.
- 8G. S. Baird, D. A. Zacharias, R. Y. Tsien, Proc. Natl. Acad. Sci. USA 2000, 97, 11984.
- 9
- 9aL. Moroder, N. Budisa, ChemPhysChem 2010, 11, 1181;
- 9bA. J. Link, D. A. Tirrell, Methods 2005, 36, 291;
- 9cN. Ayyadurai, K. Deepankumar, N. Saravanan Prabhu, S. Lee, H. Yun, Chem. Commun. 2011, 47, 3430.
- 10C. C. Liu, P. G. Schultz, Annu. Rev. Biochem. 2010, 79, 413.
- 11R. K. Boggess, R. B. Martin, J. Am. Chem. Soc. 1975, 97, 3076.
- 12
- 12aL. Alfonta, Z. Zhang, S. Uryu, J. A. Loo, P. G. Schultz, J. Am. Chem. Soc. 2003, 125, 14662;
- 12bA. Umeda, G. N. Thibodeaux, J. Zhu, Y. Lee, Z. J. Zhang, ChemBioChem 2009, 10, 1302.
- 13
- 13aS. Lepthien, L. Merkel, N. Budisa, Angew. Chem. 2010, 122, 5576;
10.1002/ange.201000439 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5446;
- 13bR. E. Doerig, B. Suter, M. Gray, E. Kubli, EMBO J. 1988, 7, 2579;
- 13cT. S. Young, I. Ahmad, J. A. Yin, P. G. Schultz, J. Mol. Biol. 2010, 395, 361.
- 14N. Ayyadurai, N. Saravanan Prabhu, K. Deepankumar, N. Soundrarajan, Y. J. Jang, N. Chitrapriya, E. Song, N. Lee, S. K. Kim, B.-G. Kim, N. Soundrarajan, S. Lee, H. J. Cha, N. Budisa, H. Yun, Bioconjug. Chem. 2011, 22, 551.
- 15Y. Rahimi, A. Goulding, S. Shrestha, S. Mirpuri, S. K. Deo, Biochem. Biophys. Res. Commun. 2008, 370, 57.
- 16P. Eli, A. Chakrabartty, Protein Sci. 2006, 15, 2442.
- 17J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed., Springer, New York, 2006.
- 18S. Nagasundarapandian, L. Merkel, N. Budisa, R. Govindan, N. Ayyadurai, S. Sriram, H. Yun, S. G. Lee, ChemBioChem 2010, 11, 2521.
- 19J. P. Sumner, N. M. Westerberg, A. K. Stoddard, T. K. Hurst, M. Cramer, R. B. Thompson, C. A. Fierke, R. Kopelman, Biosens. Bioelectron. 2006, 21, 1302.
- 20J. Lee, H. Shima, H. Choia, Y. Son, C. Lee, J. Phys. Chem. Solids 2008, 69, 1581.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.