Copper-Catalyzed 1,4-Addition of Organoboronates to Alkylidene Cyanoacetates: Mechanistic Insight and Application to Asymmetric Catalysis†
Keishi Takatsu
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorCorresponding Author
Dr. Ryo Shintani
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988Search for more papers by this authorCorresponding Author
Prof. Dr. Tamio Hayashi
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988Search for more papers by this authorKeishi Takatsu
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorCorresponding Author
Dr. Ryo Shintani
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988Search for more papers by this authorCorresponding Author
Prof. Dr. Tamio Hayashi
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988Search for more papers by this authorSupport has been provided in part by a Grant-in-Aid for Young Scientists (B), the Ministry of Education, Culture, Sports, Science, and Technology (Japan), and in part by Mitsubishi Tanabe Pharma Corporation. K.T. thanks the JSPS for a fellowship.
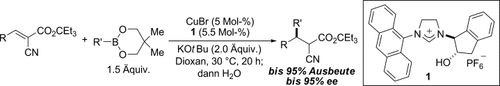
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201008196_sm_miscellaneous_information.pdf7.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see
- 1aS.-Y. Wang, T.-P. Loh, Chem. Commun. 2010, 46, 8694;
- 1bS. R. Harutyunyan, T. den Hartog, K. Geurts, A. J. Minnaard, B. L. Feringa, Chem. Rev. 2008, 108, 2824;
- 1cF. López, A. J. Minnaard, B. L. Feringa, Acc. Chem. Res. 2007, 40, 179;
- 1dA. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies, M. Diéguez, Chem. Rev. 2008, 108, 2796;
- 1eJ. Christoffers, G. Koripelly, A. Rösiak, M. Rossle, Synthesis 2007, 1279;
- 1fP. von Zezschwitz, Synthesis 2008, 1809.
- 2For recent examples, see
- 2aP. H. Bos, A. J. Minnaard, B. L. Feringa, Org. Lett. 2008, 10, 4219;
- 2bT. den Hartog, A. Rudolph, B. Maciá, A. J. Minnaard, B. L. Feringa, J. Am. Chem. Soc. 2010, 132, 14349;
- 2cJ. C. H. Lee, D. G. Hall, J. Am. Chem. Soc. 2010, 132, 5544.
- 3For recent examples, see
- 3aA. Wilsily, T. Lou, E. Fillion, Synthesis 2009, 2066;
- 3bP. H. Bos, B. Maciá, M. Á. Fernández-Ibáñez, A. J. Minnaard, B. L. Feringa, Org. Biomol. Chem. 2010, 8, 47;
- 3cL. Palais, L. Babel, A. Quintard, S. Belot, A. Alexakis, Org. Lett. 2010, 12, 1988;
- 3dK. Endo, M. Ogawa, T. Shibata, Angew. Chem. 2010, 122, 2460;
10.1002/ange.200906839 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2410.
- 4For recent examples, see
- 4aX. Tang, A. J. Blake, W. Lewis, S. Woodward, Tetrahedron: Asymmetry 2009, 20, 1881;
- 4bD. Müller, C. Hawner, M. Tissot, L. Palais, A. Alexakis, Synlett 2010, 1694;
- 4cM. Tissot, D. Müller, S. Belot, A. Alexakis, Org. Lett. 2010, 12, 2770.
- 5K. Lee, A. H. Hoveyda, J. Org. Chem. 2009, 74, 4455.
- 6For examples of copper-catalyzed carbon–carbon bond-forming reactions with organoboronic acid derivatives, see
- 6aR. Wada, K. Oisaki, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2004, 126, 8910;
- 6bR. Wada, T. Shibuguchi, S. Makino, K. Oisaki, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2006, 128, 7687;
- 6cD. Tomita, M. Kanai, M. Shibasaki, Chem. Asian J. 2006, 1, 161;
- 6dT. Ohishi, M. Nishiura, Z. Hou, Angew. Chem. 2008, 120, 5876;
10.1002/ange.200801857 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5792;
- 6eJ. Takaya, S. Tadami, K. Ukai, N. Iwasawa, Org. Lett. 2008, 10, 2697;
- 6fY. Yamamoto, N. Kirai, Y. Harada, Chem. Commun. 2008, 2010;
- 6gY. Yamamoto, N. Kirai, Org. Lett. 2008, 10, 5513;
- 6hH. Zheng, Q. Zhang, J. Chen, M. Liu, S. Cheng, J. Ding, H. Wu, W. Su, J. Org. Chem. 2009, 74, 943;
- 6iH. Ohmiya, U. Yokobori, Y. Makida, M. Sawamura, J. Am. Chem. Soc. 2010, 132, 2895;
- 6jA. M. Whittaker, R. P. Rucker, G. Lalic, Org. Lett. 2010, 12, 3216;
- 6kR. Shintani, K. Takatsu, T. Hayashi, Chem. Commun. 2010, 46, 6822.
- 7For examples of copper-catalyzed 1,4-addition of diboron compounds, see
- 7aH. Ito, H. Yamanaka, J. Tateiwa, A. Hosomi, Tetrahedron Lett. 2000, 41, 6821;
- 7bK. Takahashi, T. Ishiyama, N. Miyaura, Chem. Lett. 2000, 29, 982;
- 7cK. Takahashi, T. Ishiyama, N. Miyaura, J. Organomet. Chem. 2001, 625, 47;
- 7dS. Mun, J.-E. Lee, J. Yun, Org. Lett. 2006, 8, 4887;
- 7eJ.-E. Lee, J. Yun, Angew. Chem. 2008, 120, 151; Angew. Chem. Int. Ed. 2008, 47, 145;
- 7fX. Feng, J. Yun, Chem. Commun. 2009, 6577;
- 7gI.-H. Chen, L. Yin, W. Itano, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 11664;
- 7hJ. M. O’Brien, K. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 10630.
- 8For examples of copper-catalyzed 1,4-addition of silylboronates, see K. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 2898.
- 9Rhodium-catalyzed asymmetric 1,4-addition of aryl boronic acids to alkylidene cyanoacetates with up to 99 % ee has been reported: S. Sörgel, N. Tokunaga, K. Sasaki, K. Okamoto, T. Hayashi, Org. Lett. 2008, 10, 589.
- 10N. P. Mankad, D. S. Laitar, J. P. Sadighi, Organometallics 2004, 23, 3369.
- 11Analytical data for 5: 1H NMR ([D8]THF): δ=7.57 (s, 2 H), 7.52 (t, J=7.8 Hz, 2 H), 7.36 (d, J=7.8 Hz, 4 H), 7.01–6.96 (m, 8 H), 6.96–6.91 (m, 2 H), 4.87 (s, 1 H), 2.57 (sept, J=6.8 Hz, 4 H), 1.26–1.19 ppm (m, 33 H); 13C NMR ([D8]THF): δ=171.6, 148.0, 146.5, 135.6, 131.3, 129.4, 129.0, 128.0, 125.3, 125.2, 125.0, 75.2, 51.3, 47.8, 29.6, 29.3, 25.2, 24.0 ppm; HRMS (ESI-TOF) calcd for C47H57CuN3O2 (M+H+): 758.3741, found: 758.3739.
- 12For examples of NMR spectroscopic studies on 1,4-addition of organocuprates, see
- 12aB. Christenson, T. Olsson, C. Ullenius, Tetrahedron 1989, 45, 523;
- 12bS. H. Bertz, R. A. Smith, J. Am. Chem. Soc. 1989, 111, 8276;
- 12cS. H. Bertz, M. K. Carlin, D. A. Deadwyler, M. Murphy, C. A. Ogle, P. H. A. Seagle, J. Am. Chem. Soc. 2002, 124, 13650; see also:
- 12dK. Nilsson, C. Ullenius, N. Krause, J. Am. Chem. Soc. 1996, 118, 4194.
- 13
- 13aS. R. Harutyunyan, F. Lopéz, W. R. Browne, A. Correa, D. Peña, R. Badorrey, A. Meetsma, A. J. Minnaard, B. L. Feringa, J. Am. Chem. Soc. 2006, 128, 9103;
- 13bA. Alexakis, C. Benhaim, S. Rosset, M. Humam, J. Am. Chem. Soc. 2002, 124, 5262; see also:
- 13cK. Nakano, Y. Bessho, M. Kitamura, Chem. Lett. 2003, 32, 224.
- 14For relevant theoretical studies, see
- 14aE. Nakamura, S. Mori, K. Morokuma, J. Am. Chem. Soc. 1997, 119, 4900;
- 14bE. Nakamura, M. Yamanaka, S. Mori, J. Am. Chem. Soc. 2000, 122, 1826;
- 14cM. Yamanaka, E. Nakamura, Organometallics 2001, 20, 5675;
- 14dM. Yamanaka, E. Nakamura, J. Am. Chem. Soc. 2005, 127, 4697.
- 15For reviews, see
- 15aJ. Wencel, M. Mauduit, H. Hénon, S. Kehrli, A. Alexakis, Aldrichimica Acta 2009, 42, 43;
- 15bL. H. Gade, S. Bellemin-Laponnaz, Coord. Chem. Rev. 2007, 251, 718;
- 15cV. César, S. Bellemin-Laponnaz, L. H. Gade, Chem. Soc. Rev. 2004, 33, 619;
- 15dM. Perry, K. Burgess, Tetrahedron: Asymmetry 2003, 14, 951.
- 16For recent progress, see
- 16aR. E. Giudici, A. H. Hoveyda, J. Am. Chem. Soc. 2007, 129, 3824;
- 16bT. L. May, M. K. Brown, A. H. Hoveyda, Angew. Chem. 2008, 120, 7468; Angew. Chem. Int. Ed. 2008, 47, 7358;
- 16cY. Lee, H. Jang, A. H. Hoveyda, J. Am. Chem. Soc. 2009, 131, 18234;
- 16dK. Akiyama, F. Gao, A. H. Hoveyda, Angew. Chem. 2010, 122, 429;
10.1002/ange.200905223 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 419;
- 16eM. R. Chaulagain, G. J. Sormunen, J. Montgomery, J. Am. Chem. Soc. 2007, 129, 9568;
- 16fY.-X. Jia, J. M. Hillgren, E. L. Watson, S. P. Marsden, E. P. Kündig, Chem. Commun. 2008, 4040;
- 16gH. Hénon, M. Mauduit, A. Alexakis, Angew. Chem. 2008, 120, 9262;
10.1002/ange.200803735 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9122;
- 16hT. Uchida, T. Katsuki, Tetrahedron Lett. 2009, 50, 4741;
- 16iK. B. Selim, Y. Matsumoto, K. Yamada, K. Tomioka, Angew. Chem. 2009, 121, 8889;
10.1002/ange.200904676 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8733;
- 16jN. Shibata, M. Okamoto, Y. Yamamoto, S. Sakaguchi, J. Org. Chem. 2010, 75, 5707.
- 17For precedents of this family of chiral NHCs, see
- 17aH. Clavier, L. Coutable, J.-C. Guillemin, M. Mauduit, Tetrahedron: Asymmetry 2005, 16, 921;
- 17bH. Clavier, L. Coutable, L. Toupet, J.-C. Guillemin, M. Mauduit, J. Organomet. Chem. 2005, 690, 5237;
- 17cD. Martin, S. Kehrli, M. d′Augustin, H. Clavier, M. Mauduit, A. Alexakis, J. Am. Chem. Soc. 2006, 128, 8416;
- 17dS. Kehrli, D. Martin, D. Rix, M. Mauduit, A. Alexakis, Chem. Eur. J. 2010, 16, 9890; See also:
- 17eP. L. Arnold, M. Rodden, K. M. Davis, A. C. Scarisbrick, A. J. Blake, C. Wilson, Chem. Commun. 2004, 1612.
- 18CCDC 804933 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19
- 19aThe use of a substrate having a smaller ester group such as a methyl ester gives the product with lower enantioselectivity (e.g., 78 % yield, 76 % ee for the reaction of the methyl ester variant of 8 a with phenylboronate 2);
- 19bthe use of organoboronic acid pinacol esters instead of neopentylglycol esters results in lower enantioselectivity by ca. 10 %;
- 19cessentially the same result is obtained by conducting the reaction in the presence of tBuOH (1.0 equiv) or by using NaOtBu as the base;
- 19dsignificantly lower reactivity is observed by using KOMe as the base (e.g., 45 % yield, 92 % ee for the reaction of 8 a with phenylboronate 2), presumably because of the poor solubility of KOMe in dioxane.
- 20H. M. Davies, T. M. Gregg, Tetrahedron Lett. 2002, 43, 4951.
- 21
- 21aK. P. Bogeso, A. V. Christensen, J. Hyttel, T. Liljefors, J. Med. Chem. 1985, 28, 1817;
- 21bJ. Hyttel, J. J. Larsen, J. Neurochem. 1985, 44, 1615.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.