Octaselenocyclododecane†
Dr. Guoxiong Hua
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorDr. John M. Griffin
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorDr. Sharon E. Ashbrook
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorProf. Alexandra M. Z. Slawin
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorCorresponding Author
Prof. J. Derek Woollins
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834Search for more papers by this authorDr. Guoxiong Hua
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorDr. John M. Griffin
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorDr. Sharon E. Ashbrook
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorProf. Alexandra M. Z. Slawin
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
Search for more papers by this authorCorresponding Author
Prof. J. Derek Woollins
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834
School of Chemistry, University of St Andrews, Fife, KY16 9ST (UK), Fax: (+44) 1334-463-834Search for more papers by this authorWe are grateful to the University of St Andrews for support.
Graphical Abstract
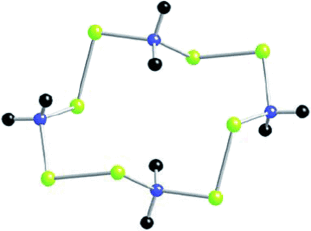
Der erste seiner Art? Ein einfach aufgebauter, zwölfgliedriger C-Se-Heterocyclus (siehe Struktur; blau C, gelb Se, schwarz H) wurde bei der Umsetzung von sekundären Aminen mit dem Woollins-Reagens erhalten. Die Bildung des Rings verläuft über intermediäre Diselenoate und erfordert ein polares Lösungsmittel wie Dichlormethan.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201006081_sm_miscellaneous_information.pdf395.9 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Topics in Current Chemistry: Organoselenium Chemistry, Modern Developments in Organic Synthesis (Ed.: ), Springer, Berlin, 2000;
- 1bT. Wirth, Angew. Chem. 2000, 112, 3890–3900;
Angew. Chem. Int. Ed. 2000, 39, 3740–3749.
10.1002/1521-3773(20001103)39:21<3740::AID-ANIE3740>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 2
- 2a Organic Selenium Compounds: Their Chemistry and Biology (Eds.: ), Wiley, New York, 1973;
- 2bK. C. Nicolaou, N. A. Petasis, Selenium in Natural Products Synthesis, CIS, Philadelphia, 1984;
- 2cG. Mugesh, W. W. Du Mont, H. Sies, Chem. Rev. 2001, 101, 2125–2179.
- 3T. G. Back, Organoselenium Chemistry. A Practical Approach, Oxford, New York, 2000.
- 4C. Höbartner, R. Micura, J. Am. Chem. Soc. 2004, 126, 1141–1149.
- 5Y. Buzin, N. Carrasco, Z. Huang, Org. Lett. 2004, 6, 1099–1102.
- 6D. M. Hatch, J. O. Boles, Z. Z. Li, L. A. Silks, Curr. Org. Chem. 2004, 8, 47–64.
- 7Y. Kobayashi, Y. Ogra, K. Ishiwata, H. Takayama, N. Aimi, K. T. Suzuki, Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936.
- 8K. EI-Bayoumy, R. Sinha, Mutat. Res. 2004, 551, 181–197.
- 9P. D. Whanger, Br. J. Nutr. 2004, 91, 11–28.
- 10G. Roy, M. Nethaji, G. Mugesh, J. Am. Chem. Soc. 2004, 126, 2712–2713.
- 11Y. T. Zhu, M. D. Gieselman, H. Zhou, O. Averin, W. A. van der Donk, Org. Biomol. Chem. 2003, 1, 3304–3315.
- 12G. Mugesh, W. W. du Mont, Chem. Eur. J. 2001, 7, 1365–1370.
10.1002/1521-3765(20010401)7:7<1365::AID-CHEM1365>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 13G. Hua, J. D. Woollins, Angew. Chem. 2009, 121, 1394–1403; Angew. Chem. Int. Ed. 2009, 48, 1368–1377.
- 14G. Hua, Y. Li, A. M. Z. Slawin, J. D. Woollins, Angew. Chem. 2008, 120, 2899–2901; Angew. Chem. Int. Ed. 2008, 47, 2857–2859.
- 15S. Tomoda, M. Iwaoka, Chem. Commun. 1990, 231–233.
- 16I. P. Gray, A. M. Z. Slawin, J. D. Woollins, Dalton Trans. 2005, 2188–2194.
- 17A. L. Fuller, L. A. S. Scott-Hayward, Y. Li, M. Bühl, A. M. Z. Slawin, J. D. Woollins, J. Am. Chem. Soc. 2010, 132, 5799–5802.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.