Strategy Towards Olefin Carbohydroxylation: Transmetalation of 2-Rhodaoxetanes with Organoboron Nucleophiles†
Alexander Dauth
Chemistry Department, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847 http://www.chem.ubc.ca/personnel/faculty/love/index.shtml
Search for more papers by this authorCorresponding Author
Prof. Jennifer A. Love
Chemistry Department, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847 http://www.chem.ubc.ca/personnel/faculty/love/index.shtml
Chemistry Department, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847 http://www.chem.ubc.ca/personnel/faculty/love/index.shtmlSearch for more papers by this authorAlexander Dauth
Chemistry Department, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847 http://www.chem.ubc.ca/personnel/faculty/love/index.shtml
Search for more papers by this authorCorresponding Author
Prof. Jennifer A. Love
Chemistry Department, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847 http://www.chem.ubc.ca/personnel/faculty/love/index.shtml
Chemistry Department, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847 http://www.chem.ubc.ca/personnel/faculty/love/index.shtmlSearch for more papers by this authorWe thank the following for support of this research: NSERC (Discovery Grant, Research Tools and Instrumentation Grants), University of British Columbia (UGF, Laird and 4YF to A.D.), and Mountain Equipment Coop (A.D.).
Graphical Abstract
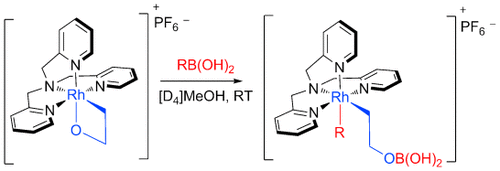
Der erste Schritt ist getan: Ein 2-Rhodaoxetan geht eine effiziente Transmetallierung mit zahlreichen funktionalisierten Aryl- und Alkenylboronsäuren ein (siehe Schema). Der Prozess ist mit diversen funktionellen Gruppen verträglich und bildet den ersten Schritt in einer postulierten katalytischen oxidativen Olefinfunktionalisierung.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201003348_sm_miscellaneous_information.pdf498.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Sawada, K. Matsumoto, T. Katsuki, Angew. Chem. 2007, 119, 4643–4645; Angew. Chem. Int. Ed. 2007, 46, 4559–4561.
- 2
- 2aJ. W. Bode, E. M. Carreira, J. Am. Chem. Soc. 2001, 123, 3611–3612;
- 2bA. Brandi, S. Cicchi, V. Paschetta, D. Gomez Pardo, J. Cossy, Tetrahedron Lett. 2002, 43, 9357–9359.
- 3T. Bach, Tetrahedron Lett. 1994, 35, 1855–1858.
- 4S. Suga, Y. Kageyama, G. Babu, K. Itami, J. Yoshida, Org. Lett. 2004, 6, 2709–2711.
- 5S. P. Miller, J. B. Morgan, F. J. V. Nepveux, J. P. Morken, Org. Lett. 2004, 6, 131–133.
- 6D. Penno, V. Lillo, I. O. Koshevoy, M. Sanaú, M. A. Ubeda, P. Lahuerta, E. Fernández, Chem. Eur. J. 2008, 14, 10648–10655.
- 7D. Jiang, J. Peng, Y. Chen, Org. Lett. 2008, 10, 1695–1698.
- 8S. Kirchberg, R. Fröhlich, A. Studer, Angew. Chem. 2009, 121, 4299–4302;
10.1002/ange.200901072 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4235–4238.
- 9Certain metal-catalyzed carbohydroxylation methods have also been reported; Pd-catalyzed carbohydroxylation of electron-deficient olefins:
- 9aH. Nakamura, M. Sekido, M. Ito, Y. Yamamoto, J. Am. Chem. Soc. 1998, 120, 6838–6839; Pt-catalyzed intramolecular carbohydroxylation:
- 9bA. Fürstner, H. Szillat, F. Stelzer, J. Am. Chem. Soc. 2000, 122, 6785–6786; Au-catalyzed oxyarylation of alkenes:
- 9cA. D. Melhado, W. E. Brenzovich, A. D. Lackner, F. D. Toste, J. Am. Chem. Soc. 2010, 132, 8885–8887.
- 10
- 10aB. de Bruin, P. H. M. Budzelaar, A. W. Gal, Angew. Chem. 2004, 116, 4236–4251; Angew. Chem. Int. Ed. 2004, 43, 4142–4157;
- 10bK. A. Joergensen, B. Schioett, Chem. Rev. 1990, 90, 1483–1506.
- 11
- 11aP. Norrby, H. Becker, K. B. Sharpless, J. Am. Chem. Soc. 1996, 118, 35–42;
- 11bE. J. Corey, M. C. Noe, J. Am. Chem. Soc. 1996, 118, 11038–11053.
- 12J. R. Khusnutdinova, L. L. Newman, P. Y. Zavalij, Y. Lam, A. N. Vedernikov, J. Am. Chem. Soc. 2008, 130, 2174–2175.
- 13
- 13aM. P. del Río, M. A. Ciriano, C. Tejel, Angew. Chem. 2008, 120, 2536–2539;
10.1002/ange.200705802 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2502–2505;
- 13bM. A. Cinellu, G. Minghetti, F. Cocco, S. Stoccoro, A. Zucca, M. Manassero, Angew. Chem. 2005, 117, 7052–7055;
10.1002/ange.200501754 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6892–6895;
- 13cE. Szuromi, J. Wu, P. R. Sharp, J. Am. Chem. Soc. 2006, 128, 12088–12089;
- 13dE. Szuromi, H. Shan, P. R. Sharp, J. Am. Chem. Soc. 2003, 125, 10522–10523;
- 13eJ. Wu, P. R. Sharp, Organometallics 2008, 27, 1234–1241.
- 14
- 14aB. Schiott, K. A. Jorgensen, J. Chem. Soc. Dalton Trans. 1993, 337–44;
- 14bC. Molinaro, T. F. Jamison, J. Am. Chem. Soc. 2003, 125, 8076–8077.
- 15
- 15aG. A. Vaughan, G. L. Hillhouse, R. T. Lum, S. L. Buchwald, A. L. Rheingold, J. Am. Chem. Soc. 1988, 110, 7215–7217;
- 15bR. Beckhaus, J. Oster, J. Sang, I. Strauss, M. Wagner, Synlett 1997, 241–249.
- 16
- 16aW. A. Herrmann, U. Kuesthardt, A. Schaefer, E. Herdtweck, Angew. Chem. 1986, 98, 818–819;
- 16bJ. F. Hartwig, R. G. Bergman, R. A. Andersen, Organometallics 1991, 10, 3344–3362;
- 16cJ. Sundermeyer, K. Weber, H. Pritzkow, Angew. Chem. 1993, 105, 751–753; Angew. Chem. Int. Ed. Engl. 1993, 32, 731–733.
- 17
- 17aB. de Bruin, M. J. Boerakker, J. J. J. M. Donners, B. E. C. Christiaans, P. P. J. Schlebos, R. de Gelder, J. M. M. Smits, A. L. Spek, A. W. Gal, Angew. Chem. 1997, 109, 2153–2157; Angew. Chem. Int. Ed. Engl. 1997, 36, 2064–2067;
- 17bB. de Bruin, M. J. Boerakker, R. de Gelder, J. M. M. Smits, A. W. Gal, Angew. Chem. 1999, 111, 118–121;
Angew. Chem. Int. Ed. 1999, 38, 219–222;
10.1002/(SICI)1521-3773(19990115)38:1/2<219::AID-ANIE219>3.0.CO;2-I CAS Web of Science® Google Scholar
- 17cB. de Bruin, M. J. Boerakker, J. A. Brands, J. J. J. M. Donners, M. P. J. Donners, R. de Gelder, J. M. M. Smits, A. W. Gal, A. L. Spek, Chem. Eur. J. 1999, 5, 2921–2936;
10.1002/(SICI)1521-3765(19991001)5:10<2921::AID-CHEM2921>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 17dB. de Bruin, M. J. Boerakker, J. A. W. Verhagen, R. de Gelder, J. M. M. Smits, A. W. Gal, Chem. Eur. J. 2000, 6, 298–312;
10.1002/(SICI)1521-3765(20000117)6:2<298::AID-CHEM298>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 17eB. de Bruin, J. A. W. Verhagen, C. H. J Schouten, A. W. Gal, D. Feichtinger, D. A. Plattner, Chem. Eur. J. 2001, 7, 416–422;
10.1002/1521-3765(20010119)7:2<416::AID-CHEM416>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 17fT. C. Flood, M. Iimura, J. M. Perotti, A. L. Rheingold, T. E. Concolino, Chem. Commun. 2000, 1681–1682.
- 18P. H. M. Budzelaar, A. N. J. Blok, Eur. J. Inorg. Chem. 2004, 2385–2391.
- 19Both the desired transmetalation product 4 and the phenolic by-product from H2O2 oxidation of the arylboronic acid are formed; this result demonstrates that sequential ethylene oxidation and transmetalation is competitive with aryl boronic acid decomposition, which bodes well for eventual development of a catalytic process. We are also assessing oxidants other than H2O2 to get maximum compatibility with the boron nucleophiles employed.
- 20J. Yu, R. Kuwano, Angew. Chem. 2009, 121, 7353–7356; Angew. Chem. Int. Ed. 2009, 48, 7217–7220.
- 21N. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483.
- 22S. Bruns, V. Sinnwell, J. Voss, Magn. Reson. Chem. 2003, 41, 269–272.
- 23
- 23aW. J. Hoogervorst, K. Goubitz, J. Fraanje, M. Lutz, A. L. Spek, J. M. Ernsting, C. J. Elsevier, Organometallics 2004, 23, 4550–4563;
- 23bT. M. Douglas, A. B. Chaplin, A. S. Weller, Organometallics 2008, 27, 2918–2921.
- 24For a more detailed discussion of this investigation including all experimental parameters and sample spectra see the supporting information.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.