Crystalline 1H-1,2,3-Triazol-5-ylidenes: New Stable Mesoionic Carbenes (MICs)†
Gregorio Guisado-Barrios Dr.
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorJean Bouffard Dr.
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorBruno Donnadieu
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorGuy Bertrand Prof.
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorGregorio Guisado-Barrios Dr.
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorJean Bouffard Dr.
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorBruno Donnadieu
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorGuy Bertrand Prof.
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorWe are grateful to the NIH (R01 GM 68825) and the DOE (DE-FG02-09ER16069) for financial support of this work.
Graphical Abstract
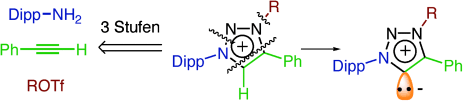
Mit einem Klick zu MICs: Eine kurze modulare Synthese führt zu neuartigen stabilen Heterocyclen mit einem freien Elektronenpaar an einem Kohlenstoffzentrum. Diese mesoionischen Verbindungen sind bessere Donoren als klassische N-heterocyclische Carbene, und sie sind durch Deprotonierung der konjugierten Säuren mit vergleichsweise milden Basen zugänglich.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201001864_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. Peris, R. H. Crabtree, C. R. Chim. 2003, 6, 33–37.
- 2
- 2aL. Tschugajeff, M. Skanawy-Grigorjewa, A. Posnjak, Z. Anorg. Allg. Chem. 1925, 148, 37–42;
- 2bK. Öfele, J. Organomet. Chem. 1968, 12, P 42-P43;
- 2cD. J. Cardin, B. Cetinkaya, M. F. Lappert, Chem. Rev. 1972, 72, 545–574.
- 3For selected recent reviews, see:
- 3aJ. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Hwang, I. J. B. Lin, Chem. Rev. 2009, 109, 3561–3598;
- 3bP. L. Arnold, I. J. Casely, Chem. Rev. 2009, 109, 3599–3611;
- 3cS. Díez-González, N. Marion, S. P. Nolan, Chem. Rev. 2009, 109, 3612–3676;
- 3dM. Poyatos, J. A. Mata, E. Peris, Chem. Rev. 2009, 109, 3677–3707;
- 3eC. Samojłowicz, M. Bieniek, K. Grela, Chem. Rev. 2009, 109, 3708–3742;
- 3fW. A. L. van Otterlo, C. B. de Koning, Chem. Rev. 2009, 109, 3743–3782;
- 3gS. Monfette, D. E. Fogg, Chem. Rev. 2009, 109, 3783–3816;
- 3hB. Alcaide, P. Almendros, A. Luna, Chem. Rev. 2009, 109, 3817–3858.
- 4For the first examples of stable carbenes, see:
- 4aA. Igau, H. Grützmacher, A. Baceiredo, G. Bertrand, J. Am. Chem. Soc. 1988, 110, 6463–6466;
- 4bA. Igau, A. Baceiredo, G. Trinquier, G. Bertrand, Angew. Chem. 1989, 101, 617–618; Angew. Chem. Int. Ed. Engl. 1989, 28, 621–622;
- 4cA. J. Arduengo III, R. L. Harlow, M. Kline, J. Am. Chem. Soc. 1991, 113, 361–363;
- 4dA. J. Arduengo III, J. R. Goerlich, W. J. Marshall, J. Am. Chem. Soc. 1995, 117, 11027–11028;
- 4eD. Enders, K. Breuer, G. Raabe, J. Runsink, J. H. Teles, J.-P. Melder, K. Ebel, S. Brode, Angew. Chem. 1995, 107, 1119–1122;
10.1002/ange.19951070926 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1021–1023.
- 5For reviews on different types of stable carbenes, see:
- 5aM. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. 2010, DOI: ; Angew. Chem. Int. Ed. 2010, DOI: ;
- 5bD. Tapu, D. A. Dixon, C. Roe, Chem. Rev. 2009, 109, 3385–3407;
- 5cJ. Vignolle, X. Cattoën, D. Bourissou, Chem. Rev. 2009, 109, 3333–3384;
- 5dF. E. Hahn, M. C. Jahnke, Angew. Chem. 2008, 120, 3166–3216; Angew. Chem. Int. Ed. 2008, 47, 3122–3172;
- 5eY. Canac, M. Soleilhavoup, S. Conejero, G. Bertrand, J. Organomet. Chem. 2004, 689, 3857–3865;
- 5fD. Bourissou, O. Guerret, F. P. Gabbaï, G. Bertrand, Chem. Rev. 2000, 100, 39–91.
- 6For recent reviews, see:
- 6aC. Grondal, M. Jeanty, D. Enders, Nat. Chem. 2010, 2, 167–178;
- 6bD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606–5655;
- 6cV. Nair, S. Vellalath, B. P. Babu, Chem. Soc. Rev. 2008, 37, 2691–2698;
- 6dN. E. Kamber, W. Jeong, R. M. Waymouth, R. C. Pratt, B. G. G. Lohmeijer, J. L. Hedrick, Chem. Rev. 2007, 107, 5813–5840.
- 7
- 7aS. Gründemann, A. Kovacevic, M. Albrecht, J. W. Faller, R. H. Crabtree, Chem. Commun. 2001, 2274–2275;
- 7bS. Gründemann, A. Kovacevic, M. Albrecht, J. W. Faller, R. H. Crabtree, J. Am. Chem. Soc. 2002, 124, 10473–10481.
- 8
- 8aY. Han, H. V. Huynh, G. K. Tan, Organometallics 2007, 26, 6581–6585;
- 8bY. Han, L. J. Lee, H. V. Huynh, Organometallics 2009, 28, 2778–2786.
- 9aP. Mathew, A. Neels, M. Albrecht, J. Am. Chem. Soc. 2008, 130, 13534–13535;
- 9bT. Karthikeyan, S. Sankararaman, Tetrahedron Lett. 2009, 50, 5834–5837.
- 10For reviews on aNHCs and rNHCs, see:
- 10aP. L. Arnold, S. Pearson, Coord. Chem. Rev. 2007, 251, 596–609;
- 10bM. Albrecht, Chem. Commun. 2008, 3601–3610;
- 10cO. Schuster, L. Yang, H. G. Raubenheimer, M. Albrecht, Chem. Rev. 2009, 109, 3445–3478;
- 10dM. Albrecht, Chimia 2009, 63, 105–110.
- 11 Compendium of Chemical Terminology, 2nd ed. ), Blackwell Scientific, Oxford, 1997. XML online corrected version: http://goldbook.iupac.org (2006) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins [http://goldbook.iupac.org/M03842.html].
- 12R. W. Alder, M. E. Blake, L. Chaker, J. N. Harvey, F. Paolini, J. Schütz, Angew. Chem. 2004, 116, 6020–6036;
10.1002/ange.200400654 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5896–5911.
- 13E. Aldeco-Perez, A. J. Rosenthal, B. Donnadieu, P. Parameswaran, G. Frenking, G. Bertrand, Science 2009, 326, 556–559.
- 14
- 14aV. Lavallo, C. A. Dyker, B. Donnadieu, G. Bertrand, Angew. Chem. 2008, 120, 5491–5494;
10.1002/ange.200801176 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5411–5414;
- 14bM. Melaimi, P. Parameswaran, B. Donnadieu, G. Frenking, G. Bertrand, Angew. Chem. 2009, 121, 4886–4889;
10.1002/ange.200901117 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4792–4795.
- 15For publications related to bent allenes, see:
- 15aM. M. Hänninen, A. Peuronen, H. M. Tuononen, Chem. Eur. J. 2009, 15, 7287–7291;
- 15bI. Fernández, C. A. Dyker, A. DeHope, B. Donnadieu, G. Frenking, G. Bertrand, J. Am. Chem. Soc. 2009, 131, 11875–11881;
- 15cC. A. Dyker, V. Lavallo, B. Donnadieu, G. Bertrand, Angew. Chem. 2008, 120, 3250–3253;
10.1002/ange.200705620 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3206–3209;
- 15dR. Tonner, G. Frenking, Angew. Chem. 2007, 119, 8850–8853;
10.1002/ange.200701632 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8695–8698;
- 15eG. Frenking, R. Tonner, Pure Appl. Chem. 2009, 81, 597–614;
- 15fA. Fürstner, M. Alcarazo, R. Goddard, C. W. Lehmann, Angew. Chem. 2008, 120, 3254–3258;
10.1002/ange.200705798 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3210–3214;
- 15gM. Alcarazo, C. W. Lehmann, A. Anoop, W. Thiel, A. Fürstner, Nat. Chem. 2009, 1, 295–301.
- 16A prior report showed that mesoionic 1H-1,2,3-triazol-5-ylidenes of type V have a sufficient lifetime in solution to participate in intermolecular reactions. M. Begtrup, J. Chem. Soc. Chem. Commun. 1975, 334–335.
- 17
- 17aC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3064;
- 17bV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708–2711;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596–2599; for a recent review,10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 17cM. Meldal, C. W. Tornøe, Chem. Rev. 2008, 108, 2952–3015.
- 18K. Barral, A. D. Moorhouse, J. E. Moses, Org. Lett. 2007, 9, 1809–1811.
- 19
- 19aV. Lavallo, Y. Ishida, B. Donnadieu, G. Bertrand, Angew. Chem. 2006, 118, 6804–6807;
10.1002/ange.200602701 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6652–6655;
- 19bV. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2006, 312, 722–724.
- 20CCDC 770957 (2 a) and CCDC 770958 (Va) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21
- 21aD. Mendozy-Espinosa, B. Donnadieu, G. Bertrand, J. Am. Chem. Soc. 2010, DOI: ;
- 21bA. J. Arduengo III, F. Davidson, H. V. R. Dias, J. R. Goerlich, D. Khasnis, W. J. Marshall, T. K. Prakasha, J. Am. Chem. Soc. 1997, 119, 12742–12749;
- 21cM. L. Cole, C. Jones, P. C. Junk, New J. Chem. 2002, 26, 1296–1303.
- 22R. A. Kelly III, H. Clavier, S. Giudice, N. M. Scott, E. D. Stevens, J. Bordner, I. Samardjiev, C. D. Hoff, L. Cavallo, S. P. Nolan, Organometallics 2008, 27, 202–210.
- 23
- 23aA. R. Chianese, A. Kovacevic, B. M. Zeglis, J. W. Faller, R. H. Crabtree, Organometallics 2004, 23, 2461–2468;
- 23bG. Song, Y. Zhang, X. Li, Organometallics 2008, 27, 1936–1943.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.