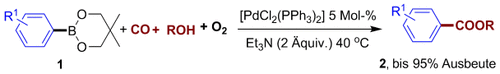Palladium-Catalyzed Aerobic Oxidative Carbonylation of Arylboronate Esters under Mild Conditions†
Qiang Liu
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorGang Li
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorJun He
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorJing Liu Dr.
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorPeng Li
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorAiwen Lei Prof.
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, 730000 Lanzhou (China)
Search for more papers by this authorQiang Liu
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorGang Li
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorJun He
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorJing Liu Dr.
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorPeng Li
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
Search for more papers by this authorAiwen Lei Prof.
The College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei (China), Fax: (+86) 27-6875-4067 http://www.chem.whu.edu.cn/GCI_WebSite/main.htm
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, 730000 Lanzhou (China)
Search for more papers by this authorThis work was supported by the National Natural Science Foundation of China (20772093, 20972118, and 20832003), and the Doctoral Fund of the Ministry of Education of China (20060486005).
Graphical Abstract
Mit CO und Luft: Die Titelreaktion mit [PdCl2(PPh3)2] als Katalysatorvorstufe ergab unter sehr milden Bedingungen (leichter Überdruck von CO und Luft, 40→50 °C) zahlreiche Arylcarbonsäureester 2 in guten bis hervorragenden Ausbeuten. Bemerkenswert war die Selektivität für die oxidative Carbonylierung gegenüber der Homokupplung der Boronsäureester 1.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201000460_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Schoenberg, R. F. Heck, J. Org. Chem. 1974, 39, 3327–3331;
- 1bA. Brennführer, H. Neumann, M. Beller, Angew. Chem. 2009, 121, 4176–4196;
10.1002/ange.200900013 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4114–4133.
- 2R. Skoda-Földes, L. Kollar, Curr. Org. Chem. 2002, 6, 1097–1119.
- 3M. Beller, B. Cornils, C. D. Frohning, C. W. Kohlpaintner, J. Mol. Catal. A 1995, 104, 17–85.
- 4G. Zanti, D. Peeters, Eur. J. Inorg. Chem. 2009, 3904–3911.
- 5C. F. J. Barnard, Org. Process Res. Dev. 2008, 12, 566–574.
- 6C. F. J. Barnard, Organometallics 2008, 27, 5402–5422.
- 7S. Inoue, H. Shiota, Y. Fukumoto, N. Chatani, J. Am. Chem. Soc. 2009, 131, 6898–6899.
- 8Z. H. Guan, Z. H. Ren, S. M. Spinella, S. C. Yu, Y. M. Liang, X. M. Zhang, J. Am. Chem. Soc. 2009, 131, 729–733.
- 9R. Giri, J. Q. Yu, J. Am. Chem. Soc. 2008, 130, 14082–14083.
- 10K. Orito, A. Horibata, T. Nakamura, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita, M. Tokuda, J. Am. Chem. Soc. 2004, 126, 14342–14343.
- 11Y. Zhao, L. Jin, P. Li, A. Lei, J. Am. Chem. Soc. 2008, 130, 9429–9433.
- 12A. Suzuki, J. Synth. Org. Chem. Jpn. 2005, 63, 312–324.
- 13A. Suzuki in Organoboranes for Syntheses, Vol. 783, American Chemical Society, New York, 2001, pp. 80–93.
10.1021/bk-2001-0783.ch006 Google Scholar
- 14 Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine (Ed.: ), Wiley-VCH, Weinheim, 2005.
- 15R. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461–1473.
- 16A. Suzuki, J. Synth. Org. Chem. Jpn. 2004, 80, 359–371.
- 17F. Bellina, A. Carpita, R. Rossi, Synthesis 2004, 2419–2440.
- 18T. Ohishi, M. Nishiura, Z. Hou, Angew. Chem. 2008, 120, 5876–5879;
10.1002/ange.200801857 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5792–5795.
- 19K. Ukai, M. Aoki, J. Takaya, N. Iwasawa, J. Am. Chem. Soc. 2006, 128, 8706–8707.
- 20H. Neumann, A. Brennfuehrer, M. Beller, Chem. Eur. J. 2008, 14, 3645–3652, and references therein.
- 21S. S. Stahl, Angew. Chem. 2004, 116, 3480–3501;
10.1002/ange.200300630 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3400–3420.
- 22J. L. Brice, J. E. Harang, V. I. Timokhin, N. R. Anastasi, S. S. Stahl, J. Am. Chem. Soc. 2005, 127, 2868–2869.
- 23M. M. Rogers, J. E. Wendlandt, I. A. Guzei, S. S. Stahl, Org. Lett. 2006, 8, 2257–2260.
- 24C. C. Scarborough, S. S. Stahl, Org. Lett. 2006, 8, 3251–3254.
- 25S. S. Stahl, Science 2005, 309, 1824–1826.
- 26V. Kotov, C. C. Scarborough, S. S. Stahl, Inorg. Chem. 2007, 46, 1910–1923.
- 27B. Gabriele, R. Mancuso, G. Salerno, E. Lupinacci, G. Ruffolo, M. Costa, J. Org. Chem. 2008, 73, 4971–4977.
- 28B. Gabriele, P. Plastina, G. Salerno, R. Mancuso, M. Costa, Org. Lett. 2007, 9, 3319–3322.
- 29B. Gabriele, G. Salerno, L. Veltri, R. Mancuso, Z. Y. Li, A. Crispini, A. Bellusci, J. Org. Chem. 2006, 71, 7895–7898.
- 30B. Gabriele, G. Salerno, M. Costa, Top. Organomet. Chem. 2006, 18, 239–272.
- 31K. Orito, M. Miyazawa, T. Nakamura, A. Horibata, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita, T. Yamazaki, M. Tokuda, J. Org. Chem. 2006, 71, 5951–5958.
- 32J. Dupont, C. S. Consorti, J. Spencer, Chem. Rev. 2005, 105, 2527–2571.
- 33At 40 °C with 5 mol % of palladium catalyst, the reactions need 48 hours to reach completion. When the reaction temperature was increased to 60 °C, low conversion and palladium-black formation were observed.
- 34Y. Izawa, I. Shimizu, A. Yamamoto, Bull. Chem. Soc. Jpn. 2004, 77, 2033–2045.
- 35C. Adamo, C. Amatore, I. Ciofini, A. Jutand, H. Lakmini, J. Am. Chem. Soc. 2006, 128, 6829–6836.
- 36This transmetalation step did not involve the ligand dissociation, see Ref. [35].
- 37J. S. Chen, K. Krogh-Jespersen, J. G. Khinast, J. Mol. Catal. A 2008, 285, 14–19.
- 38H. Yoshida, Y. Yamaryo, J. Ohshita, A. Kunai, Tetrahedron Lett. 2003, 44, 1541–1544.
- 39K. A. Smith, E. M. Campi, W. R. Jackson, S. Marcuccio, C. G. M. Naeslund, G. B. Deacon, Synlett 1997, 131–132.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.