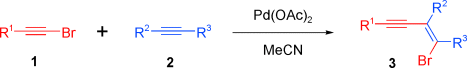Expedient Synthesis of Functionalized Conjugated Enynes: Palladium-Catalyzed Bromoalkynylation of Alkynes†
Yibiao Li
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorXiaohang Liu
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorHuanfeng Jiang Prof. Dr.
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorZhenning Feng
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorYibiao Li
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorXiaohang Liu
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorHuanfeng Jiang Prof. Dr.
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorZhenning Feng
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640 (China), Fax: (+86) 20-8711-2906
Search for more papers by this authorWe are grateful to the National Natural Foundation of China (Nos. 20625205, 20772034, and 20932002) and the Doctoral Fund of the Ministry of Education of China (No. 20090172110014) for financial support.
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201000003_sm_miscellaneous_information.pdf864 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on conjugated structures, see:
- 1aE. Negishi, L. Anastasia, Chem. Rev. 2003, 103, 1979–2017;
- 1bE. Negishi, M. Qian, F. Zeng, L. Anastasia, D. Babinski, Org. Lett. 2003, 5, 1597–1600;
- 1cK. C. Nicolaou, H. Zhang, J. S. Chen, J. J. Crawford, L. Pasunoori, Angew. Chem. 2007, 119, 4788–4791;
10.1002/ange.200700917 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4704–4707;
- 1dY. Liu, H. Gao, Org. Lett. 2006, 8, 309–311;
- 1eY. Liu, C. Xi, R. Hara, K. Nakajima, A. Yamazaki, M. Kotora, T. Takahashi, J. Org. Chem. 2000, 65, 6951–6957;
- 1fX. Huang, D. Duan, W. Zheng, J. Org. Chem. 2003, 68, 1958–1963;
- 1gS. M. Lee, K. H. Bae, H. J. Sohn, Tetrahedron Lett. 2009, 50, 416–418;
- 1hX. Zeng, F. Zeng, E. Negishi, Org. Lett. 2004, 6, 3245–3248;
- 1iZ. U. Levi, T. D. Tilley, J. Am. Chem. Soc. 2009, 131, 2796–2797;
- 1jA. S. Andersson, L. Kerndrup, A. ø. Madsen, K. Kilså, M. B. Nielsen, J. Org. Chem. 2009, 74, 375–382;
- 1kX. Nie, G. Wang, J. Org. Chem. 2006, 71, 4734–4741.
- 2
- 2aG. T. Hwang, H. S. Son, J. K. Ku, B. H. Kim, J. Am. Chem. Soc. 2003, 125, 11241–11248;
- 2bJ. A. McCubbin, M. L. Maddess, M. Lautens, Org. Lett. 2006, 8, 2993–2996;
- 2cT. Ljungdahl, T. Bennur, A. Dallas, H. Emtenås, J. Mårtensson, Organometallics 2008, 27, 2490–2498;
- 2dM. Feuerstein, L. Chahen, H. Doucet, M. Santelli, Tetrahedron Lett. 2006, 62, 112–120;
- 2eF. Bellina, E. Falchi, R. Rossi, Tetrahedron 2003, 59, 9091–9100.
- 3
- 3aY. Nakao, Y. Hirata, M. Tanaka, T. Hiyama, Angew. Chem. 2008, 120, 391–393; Angew. Chem. Int. Ed. 2008, 47, 385–387;
- 3bE. Shirakawa, H. Yoshida, T. Kurahashi, Y. Nakao, T. Hiyama, J. Am. Chem. Soc. 1998, 120, 2975–2976;
- 3cM. Suginome, M. Shirakura, A. Yamamoto, J. Am. Chem. Soc. 2006, 128, 14438–14439;
- 3dY. Liu, Z. Zhong, K. Nakajima, T. Takahashi, J. Org. Chem. 2002, 67, 7451–7456;
- 3eA. N. Thadani, V. H. Rawal, Org. Lett. 2002, 4, 4321–4323.
- 4For recent examples of transition metal catalyzed alkynes–alkynes coupling, see:
- 4aB. M. Trost, N. Maulide, M. T. Rudd, J. Am. Chem. Soc. 2009, 131, 420–421;
- 4bB. M. Trost, A. J. Frontier, J. Am. Chem. Soc. 2000, 122, 11727–11728;
- 4cK. Ogata, J. Sugasawa, S. Fukuzawa, Angew. Chem. 2009, 121, 6194–6196;
10.1002/ange.200902099 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6078–6080;
- 4dH. Katayama, H. Yari, M. Tanaka, F. Ozawa, Chem. Commun. 2005, 4336–4338;
- 4eT. Nishimura, X. X. Guo, K. Ohnishi, T. Hayashi, Adv. Synth. Catal. 2007, 349, 2669–2672;
- 4fN. Tsukada, S. Ninomiya, Y. Aoyama, Y. Inoue, Org. Lett. 2007, 9, 2919–2921.
- 5H. Jiang, X. Liu, L. Zhou, Chem. Eur. J. 2008, 14, 11305–11309.
- 6
- 6aY. Takayama, C. Delas, K. Muraoka, F. Sato, Org. Lett. 2003, 5, 365–368;
- 6bM. Kivala, F. Mitzel, C. Boudon, J. P. Gisselbrecht, P. Seiler, M. Gross, F. Diederich, Chem. Asian J. 2006, 1, 479–489.
- 7
- 7aX. Zeng, M. Qian, Q. Hu, E. Negishi, Angew. Chem. 2004, 116, 2309–2313; Angew. Chem. Int. Ed. 2004, 43, 2259–2263;
- 7bL. Yu, B. Meng, X. Huang, J. Org. Chem. 2008, 73, 6895–6898;
- 7cS. H. Sim, H. J. Park, S. I. Lee, Y. K. Chung, Org. Lett. 2008, 10, 433–436;
- 7dL. Xiong, M. Shi, Tetrahedron 2007, 63, 11938–11942;
- 7eY. Shibata, Y. Otake, M. Hirano, K. Tanaka, Org. Lett. 2009, 11, 689–692;
- 7fP. Bichler, W. A. Chalifoux, S. Eisler, A. L. K. S. Shun, E. T. Chernick, R. R. Tykwinski, Org. Lett. 2009, 11, 519–522.
- 8
- 8aY. Liu, F. Song, L. Cong, J. Org. Chem. 2005, 70, 6999–7002;
- 8bS. Guo, H. Zhang, F. Song, Y. Liu, Tetrahedron 2007, 63, 2009–2018.
- 9NOESY spectra of 3 aa, 3 ad; HMBC, HSQC, and ROESY spectra of 3 ah; HMBC spectrum of 4 aa are shown in the Supporting Information.
- 10
- 10aD. Sud, T. J. Wigglesworth, N. R. Branda, Angew. Chem. 2007, 119, 8163–8165;
10.1002/ange.200703034 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8017–8019;
- 10bJ. Barluenga, D. de Sáa, A. Gómez, A. Ballesteros, J. Santamaría, A. de Prado, M. Tomás, A. L. Suárez-Sobrino, Angew. Chem. 2008, 120, 6321–6324;
10.1002/ange.200801584 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6225–6228;
- 10cL. Feng, A. Zhang, S. M. Kerwin, Org. Lett. 2006, 8, 1983–1986;
- 10dH. H. Jeon, J. B. Son, J. H. Choi, I. H. Jeong, Tetrahedron Lett. 2007, 48, 627–631.
- 11The major by-product is 1,4-diphenylbuta-1,3-diyne from self-coupling of the phenylethynyl iodine. See: S. V. Damle, D. Seomoon, P. H. Lee, J. Org. Chem. 2003, 68, 7085–7087.
- 12For regioselective palladation of alkynes by palladium salts, see:
- 12aK. Kaneda, T. Uchiyama, Y. Fujiwara, T. Imanaka, S. Teranishi, J. Org. Chem. 1978, 44, 55–63;
- 12bS. Ma, X. Lu, J. Chem. Soc. Chem. Commun. 1990, 733–734;
- 12cS. Ma, X. Lu, Z. Li, J. Org. Chem. 1992, 57, 709–713.
- 13
- 13aG. Yin, G. Liu, Angew. Chem. 2008, 120, 5522–5525; Angew. Chem. Int. Ed. 2008, 47, 5442–5445;
- 13bK. M. Gericke, D. I. Chai, N. Bieler, M. Lautens, Angew. Chem. 2009, 121, 1475–1479; Angew. Chem. Int. Ed. 2009, 48, 1447–1451;
- 13cB. M. Trost, C. Chan, G. Ruhter, J. Am. Chem. Soc. 1987, 109, 3486–3487;
- 13dF. Kessler, N. Szesni, K. Põhako, B. Weibert, H. Fischer, Organometallics 2009, 28, 348–354;
- 13eK. Muñiz, Angew. Chem. 2009, 121, 9576–9588;
10.1002/ange.200903671 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9412–9423.
- 14A. J. Canty, T. Rodemann, B. W. Skelton, A. H. White, Organometallics 2006, 25, 3996–4001.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.