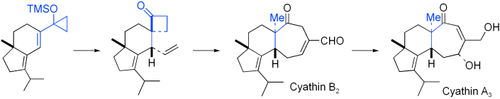
Total Synthesis of Cyathin A3 and Cyathin B2†
Keunho Kim
Department of Chemistry, Wayne State University, Detroit, MI 48236 (USA), Fax: (+1) 313-577-8822
Search for more papers by this authorJin Kun Cha Prof. Dr.
Department of Chemistry, Wayne State University, Detroit, MI 48236 (USA), Fax: (+1) 313-577-8822
Search for more papers by this authorKeunho Kim
Department of Chemistry, Wayne State University, Detroit, MI 48236 (USA), Fax: (+1) 313-577-8822
Search for more papers by this authorJin Kun Cha Prof. Dr.
Department of Chemistry, Wayne State University, Detroit, MI 48236 (USA), Fax: (+1) 313-577-8822
Search for more papers by this authorWe thank the NIH (GM 35956) for generous financial support. We also thank Dr. H. Oh for preliminary studies.
Abstract
Stereoselektiv verläuft die hier vorgestellte Synthese von Cyathin A3 und Cyathin B2 mithilfe einer Prins-Reaktion eines Cycloalkenylcyclopropanols. Erwähnung verdient auch die Anwendung einer Spirocyclobutanon-Einheit, um in einer effizienten Ringschlussmetathese stereoselektiv zu einem Siebenring mit dem gewünschten Funktionalisierungsmuster zu gelangen (siehe Schema).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200901669_sm_miscellaneous_information.pdf11.4 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. A. Ayer, H. Taube, Tetrahedron Lett. 1972, 13, 1917;
- 1bW. A. Ayer, H. Taube, Can. J. Chem. 1973, 51, 3842;
- 1cW. A. Ayer, T. T. Nakashima, D. E. Ward, Can. J. Chem. 1978, 56, 2197;
- 1dA. D. Allbutt, W. A. Ayer, H. J. Brodie, B. N. Johri, H. Taube, Can. J. Microbiol. 1971, 17, 1401.
- 2W. A. Ayer, S. P. Lee, Can. J. Chem. 1979, 57, 3332.
- 3See, for example:
- 3aH. Kawagishi, A. Shimada, R. Shirai, K. Okamoto, F. Ojima, H. Sakamoto, Y. Ishiguro, S. Furukawa, Tetrahedron Lett. 1994, 35, 1569;
- 3bT. Kita, Y. Takaya, Y. Oshima, T. Ohta, K. Aizawa, T. Hirano, T. Inakuma, Tetrahedron 1998, 54, 11877;
- 3cY. Obara, H. Kobayashi, T. Ohta, Y. Ohizumi, N. Nakahata, Mol. Pharmacol. 2001, 59, 1287;
- 3dY. Obara, T. Hoshino, M. C. Marcotullio, R. Pagiotti, N. Nakahata, Life Sci. 2007, 80, 1669.
- 4Allocyathin B2 and erinacine A:
- 4aB. B. Snider, N. H. Vo, S. V. O’Neil, B. M. Foxman, J. Am. Chem. Soc. 1996, 118, 7644;
- 4bB. B. Snider, N. H. Vo, S. V. O’Neil, J. Org. Chem. 1998, 63, 4732;
- 4cM. Tori, N. Toyoda, M. Sono, J. Org. Chem. 1998, 63, 306;
- 4dM. Takano, A. Umino, M. Nakada, Org. Lett. 2004, 6, 4897;
- 4eB. M. Trost, L. Dong, G. M. Schroeder, J. Am. Chem. Soc. 2005, 127, 2844;
- 4fB. M. Trost, L. Dong, G. M. Schroeder, J. Am. Chem. Soc. 2005, 127, 10259.
- 5Sarcodonin G: E. Piers, M. Gilbert, K. L. Cook, Org. Lett. 2000, 2, 1407.
- 6Scabronine G:
- 6aS. P. Waters, Y. Tian, Y.-M. Li, S. J. Danishefsky, J. Am. Chem. Soc. 2005, 127, 13514; see also:
- 6bR. M. Wilson, S. J. Danishefsky, Acc. Chem. Res. 2006, 39, 539.
- 7Cyanthiwigins:
- 7aM. W. B. Pfeiffer, A. J. Phillips, J. Am. Chem. Soc. 2005, 127, 5334;
- 7bT. J. Reddy, G. Bordeau, L. Trimble, Org. Lett. 2006, 8, 5585;
- 7cJ. A. Enquist, Jr., B. M. Stoltz, Nature 2008, 453, 1228; see also:
- 7dL. C. Miller, J. M. Ndungu, R. Sarpong, Angew. Chem. 2009, 121, 2434;
10.1002/ange.200806154 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2398.
- 8Allocyathin B3:
- 8aD. E. Ward, Y. Gai, Q. Qiao, Org. Lett. 2000, 2, 2125;
- 8bD. E. Ward, Y. Gai, Q. Qiao, J. Shen, Can. J. Chem. 2004, 82, 254; cyathin A3:
- 8cD. E. Ward, J. Shen, Org. Lett. 2007, 9, 2843.
- 9Erinacine B:
- 9aH. Watanabe, M. Takano, A. Umino, T. Ito, H. Ishikawa, M. Nakada, Org. Lett. 2007, 9, 359; erinacine E:
- 9bH. Watanabe, M. Nakada, J. Am. Chem. Soc. 2008, 130, 1150.
- 10
- 10aB. B. Snider, D. J. Rodini, J. van Straten, J. Am. Chem. Soc. 1980, 102, 5872;
- 10bL. A. Paquette, T. M. Heidelbaugh, Synthesis 1998, 495.
- 11For reviews, see:
- 11aO. G. Kulinkovich, A. de Meijere, Chem. Rev. 2000, 100, 2789;
- 11bO. G. Kulinkovich, Russ. Chem. Bull. Int. Ed. 2004, 53, 1065.
- 12For recent stereochemical studies, see:
- 12aI. L. Lysenko, H.-S. Oh, J. K. Cha, J. Org. Chem. 2007, 72, 7903;
- 12bWe thank Dr. Mary Jane Heeg for single crystal X-ray analysis. CCDC 230110(12β) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13For recent reviews on RCM, see: Handbook of Metathesis, Vol. 1–3 (Ed.: ), Wiley-VCH, Weinheim, 2003.
- 14For reviews, see:
- 14aF. Sato, H. Urabe, S. Okamoto, Chem. Rev. 2000, 100, 2835;
- 14bF. Sato, H. Urabe in Titanium and Zirconium in Organic Synthesis (Ed.: ), Wiley-VCH, New York, 2002, pp. 319–354.
10.1002/3527600671.ch9 Google Scholar
- 15J. Lee, J. K. Cha, Tetrahedron Lett. 1996, 37, 3663.
- 16
- 16aR. C. Krug, T. F. Yen, J. Org. Chem. 1956, 21, 1082;
- 16bT. M. Bertolini, Q. H. Nguyen, D. F. Harvey, J. Org. Chem. 2002, 67, 8675.
- 17
- 17aS. J. Cristol, K. L. Nagpal, J. Org. Chem. 1961, 26, 365;
- 17bA. A. Oswald, K. Griesbaum, W. A. Thaler, B. E. Hudson, Jr., J. Am. Chem. Soc. 1962, 84, 3897;
- 17cP. A. Wender, J. J. Howbert, Tetrahedron Lett. 1982, 23, 3983.
- 18B. H. McKee, D. G. Gilheany, K. B. Sharpless, Org. Synth. Coll. Vol. 1998, 9, 383.
- 19For recent reviews, see:
- 19aA. Padwa, D. E. Gunn, Jr., M. H. Osterhout, Synthesis 1997, 1353;
- 19bS. K. Bur, A. Padwa, Chem. Rev. 2004, 104, 2401.
- 20The [2,3]-sigmatropic rearrangement of 18 in the presence of a thiophile (e.g., trimethylphosphite) gave a 3:1 mixture of two allylic alcohols in quantitative yield: the α-alcohol exists predominantly as the hemiacetal.
- 21L. C. Vishwakarma, O. D. Stringer, F. A. Davis, Org. Synth. Coll. Vol. 1993, 8, 546.
- 22The geometry of the double bond of the vinyl sulfides was determined by NOE measurements.
- 23The configuration of the C11 alcohol was tentatively assigned by the known preference of cyathin A3 for the hemiacetal form and was also confirmed by X-ray analysis (Dr. Mary Jane Heeg) of a related compound. CCDC 640599 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 24Stereoselective reduction of 22 was achieved by L-Selectride to afford the corresponding β-alcohol at C14, the spectroscopic data of which were in excellent agreement with those of Nakada and co-workers.[9] Interestingly, this stereochemical outcome contrasts with the Nakada procedure under the control of the Corey–Bakshi–Shibata reagent.[25] Reduction of 2 with an excess of L-selectride gave small amounts of the corresponding β-diol at C14, along with large amounts of the primary alcohol (without ketone reduction; see Scheme 4). Reduction of 2 with diisobutylaluminum hydride gave a 1:3 mixture of the corresponding β- and α-diol, respectively.
- 25The enantiopure β-alcohol obtained from 22 was recently converted into (−)-3 and (−)-4 by Nakada and co-workers.[9] Albeit our alcohol product is racemic, this work can be viewed as a formal synthesis of 3 and 4.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.