Challenging the Metallocene Dominance in Actinide Chemistry with a Soft PNP Pincer Ligand: New Uranium Structures and Reactivity Patterns†
Thibault Cantat Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorChristopher R. Graves Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorBrian L. Scott Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorJaqueline L. Kiplinger Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorThibault Cantat Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorChristopher R. Graves Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorBrian L. Scott Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorJaqueline L. Kiplinger Dr.
Los Alamos National Laboratory, Mail Stop J514, Los Alamos, NM 87545 (USA), Fax: (+1) 505-667-9905
Search for more papers by this authorWe acknowledge LANL (Director's PD Fellowships to T.C. and C.R.G.), G.T. Seaborg Institute for Transactinium Science (PD Fellowship to C.R.G.), and the Heavy Element Chemistry Program, Division of Chemical Sciences, Office of Basic Energy Sciences for financial support of this work. We thank Anthony Mancinco for the design of the inside cover art.
Abstract
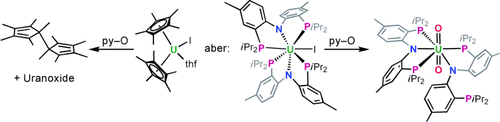
U wie ungewohnt: Der Austausch von C5Me5 gegen einen „weichen“ PNP- Pinzettenliganden mit besonderen elektronischen und sterischen Eigenschaften erzeugte neue Reaktivitäts- und Strukturmuster in der Actinoidenreihe (siehe Bild, py–O=Pyridin-N-oxid). Die neuen Liganden unterstützen nicht nur niedervalente Uranverbindungen, sondern auch hochvalente Uran(VI)-Spezies.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200806115_sm_miscellaneous_information.pdf185.2 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. J. Marks, A. Streitwieser in The Chemistry of the Actinide Elements, Vol. 2 (Eds.: ), Chapman and Hall, New York, 1986, pp. 1547–1587;
10.1007/978-94-009-3155-8_15 Google Scholar
- 1bC. J. Burns, M. S. Eisen in The Chemistry of the Actinide and Transactinide Elements, Vol. 5 (Eds.: ), Springer, Dordrecht, 2006, pp. 2799–2910.
10.1007/1-4020-3598-5_25 Google Scholar
- 2For key references on the decomposition of low-valent uranium–bis(C5Me5) complexes in oxidation reactions, see:
- 2aP. B. Duval, C. J. Burns, D. L. Clark, D. E. Morris, B. L. Scott, J. D. Thompson, E. L. Werkema, L. Jia, R. A. Andersen, Angew. Chem. 2001, 113, 3461–3465;
10.1002/1521-3757(20010917)113:18<3461::AID-ANGE3461>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3357–3361;10.1002/1521-3773(20010917)40:18<3357::AID-ANIE3357>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 2bJ. Maynadie, J.-C. Berthet, P. Thuery, M. Ephritikhine, Chem. Commun. 2007, 486–488.
- 3
- 3aO. V. Ozerov in The Chemistry of Pincer Compounds (Eds.: ), Elsevier Science, Amsterdam, 2007, p. 287;
10.1016/B978-044453138-4/50014-3 Google Scholar
- 3bJ. D. Masuda, K. C. Jantunen, O. V. Ozerov, K. J. T. Noonan, D. P. Gates, B. L. Scott, J. L. Kiplinger, J. Am. Chem. Soc. 2008, 130, 2408–2409;
- 3cJ. Scott, F. Basuli, A. R. Fout, J. C. Huffman, D. J. Mindiola, Angew. Chem. 2008, 120, 8630–8633; Angew. Chem. Int. Ed. 2008, 47, 8502–8505.
- 4Actinide–bis(C5Me5) complexes adopt a bent metallocene structural motif, with the exception of a few recent examples of uranium linear metallocene complexes, see:
- 4aA. Maynadié, J. C. Berthet, P. Thuery, M. Ephritikhine, J. Am. Chem. Soc. 2006, 128, 1082–1083;
- 4bJ. Maynadié, N. Barros, J.-C. Berthet, P. Thuery, L. Maron, M. Ephritikhine, Angew. Chem. 2007, 119, 2056–2058;
10.1002/ange.200604114 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2010–2012.
- 5T. Cantat, B. L. Scott, O. V. Ozerov, D. E. Morris, J. L. Kiplinger, Inorg. Chem. 2009, 48, 2114–2127.
- 6
- 6aB. P. Warner, B. L. Scott, C. J. Burns, Angew. Chem. 1998, 110, 1005–1007;
10.1002/(SICI)1521-3757(19980403)110:7<1005::AID-ANGE1005>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 1998, 37, 959–960;10.1002/(SICI)1521-3773(19980420)37:7<959::AID-ANIE959>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 6bJ. M. Manriquez, P. J. Fagan, T. J. Marks, S. H. Vollmer, C. S. Day, V. W. Day, J. Am. Chem. Soc. 1979, 101, 5075–5078;
- 6cP. J. Fagan, J. M. Manriquez, T. J. Marks, C. S. Day, S. H. Vollmer, V. W. Day, Organometallics 1982, 1, 170–180.
- 7Hydrazonido [η2-(N,N′)-N-NCR2]2− complexes are only known for the transition metals, in these complexes the ligand is always bridging. For representative examples, see:
- 7aL. K. Bell, W. A. Herrmann, G. W. Kriechbaum, H. Pfisterer, M. L. Ziegler, J. Organomet. Chem. 1982, 240, 381–393;
- 7bS. Gambarotta, C. Floriani, A. Chiesivilla, C. Guastini, J. Am. Chem. Soc. 1983, 105, 7295–7301;
- 7cD. Nucciarone, N. J. Taylor, A. J. Carty, Organometallics 1986, 5, 2565–2567;
- 7dW. A. Herrmann, B. Menjon, E. Herdtweck, Organometallics 1991, 10, 2134–2141;
- 7eV. Tabernero, T. Cuenca, E. Herdtweck, J. Organomet. Chem. 2002, 663, 173–182;
- 7fR. J. Wright, M. Brynda, J. C. Fettinger, A. R. Betzer, P. P. Power, J. Am. Chem. Soc. 2006, 128, 12498–12509.
- 8
- 8aD. S. J. Arney, C. J. Burns, J. Am. Chem. Soc. 1995, 117, 9448–9460;
- 8bC. R. Graves, P. Yang, S. A. Kozimor, A. E. Vaughn, D. L. Clark, S. D. Conradson, E. J. Schelter, B. L. Scott, J. D. Thompson, P. J. Hay, D. E. Morris, J. L. Kiplinger, J. Am. Chem. Soc. 2008, 130, 5272–5285.
- 9S. Patai, The Chemistry of Diazonium and Diazo Groups, Parts 1 and 2, Wiley, New York, 1978.
10.1002/9780470771549 Google Scholar
- 10O. P. Lam, P. L. Feng, F. W. Heinemann, J. M. O'Connor, K. Meyer, J. Am. Chem. Soc. 2008, 130, 2806–2816.
- 11J. L. Kiplinger, D. E. Morris, B. L. Scott, C. J. Burns, Chem. Commun. 2002, 30–31.
- 12The solution magnetic moment of 4 was determined in C6D6 (2.99 μB) by using Evans' method and confirmed the UIV oxidation state of the metal center. M. L. Naklicki, C. A. White, L. L. Plante, C. E. B. Evans, R. J. Crutchley, Inorg. Chem. 1998, 37, 1880–1885.
- 13Retention of the κ3-(P,N,P′) and κ2-(P,N) coordination modes for the two PNP ligands in 4 in solution was demonstrated by using 1H and 31P NMR spectroscopy.
- 14A Cambridge Structural Database search identified four bis(C5Me5)–uranium(VI) imido structures with an average UN bond length of 1.98 Å (range 0.09 Å). For specific references, see:
- 14a[(C5Me5)2U(NPh)2], U–N=1.952(7) Å: D. S. J. Arney, C. J. Burns, D. C. Smith, J. Am. Chem. Soc. 1992, 114, 10068–10069;
- 14b[(C5Me5)2U(NAd)2] (Ad=1-adamantyl), U–N=1.94(2), 1.96(2) Å: reference [6a];
- 14c[(C5Me5)2U(O)(N-2,6-iPr2C6H3)], U–N=1.988(4) Å: D. S. J. Arney, C. J. Burns, J. Am. Chem. Soc. 1993, 115, 9840–9841;
- 14d[(C5Me5)2U(NNCPh2)(N-2,4,6-tBu3-C6H2)], U–N=2.031(6), 1.987(5) Å: reference [11].
- 15Complex 7 was independently synthesized (isolated in 100 % yield) by oxidation of 1 with iodine. See the Supporting Information for further details.
- 16T. W. Hayton, J. M. Boncella, B. L. Scott, E. R. Batista, P. J. Hay, J. Am. Chem. Soc. 2006, 128, 10549–10559.
- 17J. J. Katz, L. R. Morss, G. T. Seaborg, Summary and Comparative Aspects of the Actinide Elements, Chapman & Hall, London, 1986.
10.1007/978-94-009-4077-2 Google Scholar
- 18L. Natrajan, F. Burdet, J. Pecaut, M. Mazzanti, J. Am. Chem. Soc. 2006, 128, 7152–7153.
- 19D. J. Mindiola, Angew. Chem. 2008, 120, 1580–1583; Angew. Chem. Int. Ed. 2008, 47, 1557–1559.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.