Total Synthesis of (+)-Isomigrastatin†
Isaac J. Krauss Dr.
The Laboratory for Bioorganic Chemistry, Sloan-Kettering Institute for Cancer Research, 1275 York Avenue, New York, NY 10021, USA, Fax: (+1) 212-772-8691
Search for more papers by this authorMihirbaran Mandal Dr.
Schering-Plough Research Institute, 2015 Galloping Hill Road, Kenilworth, NJ 07033, USA
Search for more papers by this authorSamuel J. Danishefsky Prof.
The Laboratory for Bioorganic Chemistry, Sloan-Kettering Institute for Cancer Research, 1275 York Avenue, New York, NY 10021, USA, Fax: (+1) 212-772-8691
The Department of Chemistry, Columbia University, Havemeyer Hall, New York, NY 10027, USA
Search for more papers by this authorIsaac J. Krauss Dr.
The Laboratory for Bioorganic Chemistry, Sloan-Kettering Institute for Cancer Research, 1275 York Avenue, New York, NY 10021, USA, Fax: (+1) 212-772-8691
Search for more papers by this authorMihirbaran Mandal Dr.
Schering-Plough Research Institute, 2015 Galloping Hill Road, Kenilworth, NJ 07033, USA
Search for more papers by this authorSamuel J. Danishefsky Prof.
The Laboratory for Bioorganic Chemistry, Sloan-Kettering Institute for Cancer Research, 1275 York Avenue, New York, NY 10021, USA, Fax: (+1) 212-772-8691
The Department of Chemistry, Columbia University, Havemeyer Hall, New York, NY 10027, USA
Search for more papers by this authorSupport for this work was provided by the National Institutes of Health (5 R01 CA103823) I.J.K. is grateful for an NIH postdoctoral fellowship (5 F32 AI063976). M.M. is grateful for a US Army grant (DAMD 17–03-1-0444). The authors thank Prof. Ben Shen and members of his group for helpful discussions, spectra, and an authentic sample for comparison. We also thank Dr. Luis Todaro, who is supported by the NIH-RCMI (RR-03037), for X-ray crystallographic work.
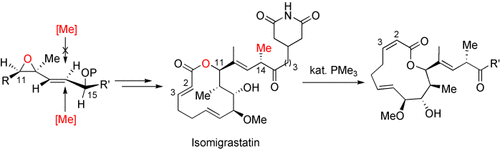
Graphical Abstract
Naturstoffe mit geringer Stabilität: Die asymmetrische Totalsynthese des hydrolytisch und thermisch labilen Naturstoffs (+)-Isomigrastatin wurde demonstriert. Die thermodynamische Instabilität der 2E-konfigurierten Doppelbindung in diesem 12-gliedrigen Makrolid wurde durch eine Phosphan-katalysierte Isomerisierung zur 2Z-Konfiguration aufgezeigt.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701837_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. J. Woo, C. M. Starks, J. R. Carney, R. Arslanian, L. Cadapan, S. Zavala, P. Licari, J. Antibiot. 2002, 55, 141.
- 2
- 2aK. Nakae, Y. Yoshimoto, T. Sawa, Y. Homma, M. Hamada, T. Takeuchi, M. Imoto, J. Antibiot. 2000, 53, 1130;
- 2bK. Nakae, Y. Yoshimoto, M. Ueda, T. Sawa, Y. Takahashi, H. Naganawa, T. Takeuchi, M. Imoto, J. Antibiot. 2000, 53, 1228;
- 2cY. Takemoto, K. Nakae, M. Kawatani, Y. Takahashi, H. Naganawa, M. Imoto, J. Antibiot. 2001, 54, 1104;
- 2dH. Nakamura, Y. Takahashi, H. Naganawa, K. Nakae, M. Imoto, M. Shiro, K. Matsumura, H. Watanabe, T. Kitahara, J. Antibiot. 2002, 55, 442.
- 3
- 3aC. Gaul, J. T. Njardarson, S. J. Danishefsky, J. Am. Chem. Soc. 2003, 125, 6042–6043;
- 3bC. Gaul, S. J. Danishefsky, Tetrahedron Lett. 2002, 43, 9039–9042.
- 4
- 4aD. Shan, L. Chen, J. T. Njardarson, C. Gaul, X. Ma, S. J. Danishefsky, X.-Y. Huang, Proc. Natl. Acad. Sci. USA 2005, 102, 3772–3776;
- 4bC. Gaul, J. T. Njardarson, D. Shan, D. C. Dorn, K. D. Wu, W. P. Tong, X. Y. Huang, M. A. S. Moore, S. J. Danishefsky, J. Am. Chem. Soc. 2004, 126, 11326–11337;
- 4cJ. T. Gaul, C. Njardarson, D. Shan, X. Y. Huang, S. J. Danishefsky, J. Am. Chem. Soc. 2004, 126, 1038–1040.
- 5aJ. H. Ju, S. K. Lim, H. Jiang, B. Shen, J. Am. Chem. Soc. 2005, 127, 1622–1623;
- 5bJ. H. Ju, S. K. Lim, H. Jiang, J. W. Seo, B. Shen, J. Am. Chem. Soc. 2005, 127, 11930–11931;
- 5cJ. H. Ju, S. K. Lim, H. Jiang, J. W. Seo, Y. Her, B. Shen, Org. Lett. 2006, 8, 5865–5868.
- 6S. J. Danishefsky, J. Schkeryantz, Synlett 1995, 475–490.
- 7A. L. Gemal, J. L. Luche, J. Am. Chem. Soc. 1981, 103, 5454–5459.
- 8R. J. Ferrier, J. Chem. Soc. 1964, 5443.
- 9A trace quantity of the α-oxiranyl β-anomeric lactol was also observed (see Supporting Information).
- 10D. B. Denney, J. Song, J. Org. Chem. 1964, 29, 495.
- 11Y. Egawa, T. Okuda, M. Suzuki, Chem. Pharm. Bull. 1963, 11, 589.
- 12There is precedent for Wittig and Horner–Emmons couplings directly with 2,3-epoxylactols: see J. E. Harvey, S. A. Raw, R. J. K. Taylor, Org. Lett. 2004, 6, 2611–2614. The difficulty in our case may have been due to the presence of an additional methyl group adjacent to the anomeric carbon center.
- 13
- 13aJ. A. Marshall, J. D. Trometer, B. E. Blough, T. D. Crute, J. Org. Chem. 1988, 53, 4274–4282;
- 13bJ. A. Marshall, B. E. Blough, J. Org. Chem. 1990, 55, 1540–1547;
- 13cJ. A. Marshall, B. E. Blough, J. Org. Chem. 1991, 56, 2225–2234. A substantial matched–mismatched effect is seen in Marshall's example (below). In the case of 20, the mismatch is enough to overturn selectivity to a syn SN2′ result, possibly owing to the larger size of the glutarimide appendage (R′, Scheme 3) relative to the methyl group in Marshall's system.
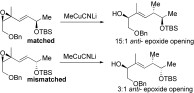
- 14Compound 20 was subjected to the cuprate SN2′ reaction as a mixture with 19, from which it was virtually inseparable. However, the corresponding products 21 and 22 were easily separated and isolated with a combined yield of 75 %.
- 15
- 15aE. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 5551–5553;
- 15bE. J. Corey, C. J. Helal, Angew. Chem. 1998, 110, 2092–2118;
10.1002/(SICI)1521-3757(19980803)110:15<2092::AID-ANGE2092>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1986–2012;10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 15cE. J. Corey, C. K. Bakshi, S. Shibata, C. P. Chen, V. K. Singh, J. Am. Chem. Soc. 1987, 109, 7925–7926.
- 16Numerous ring-closing metathesis strategies failed to give the required (2,3),(6,7)-dienolide directly, whether closure was attempted at the 2,3- or 6,7-positions. This was likely due to decomposition of this unstable motif under metathesis conditions.
- 17See the Supporting Information for preparation of 24.
- 18Integration of crude 1HNMR signals showed a 1.6:1 ratio favoring the Z geometry at C6–C7 for each C2 epimer. Future efforts will be directed toward enhancement of yield and selectivity. For a promising direction, see: A. Fürstner, K. Radkowski, C Wirtz, R. Goddard, C. W. Lehmann, R. Mynott, J. Am. Chem. Soc. 2002, 124, 7061–7069.
- 19The major C2 epimer eliminated along C2–C3 to give a separable 9:1 mixture of E and Z products. The minor C2 epimer afforded exclusively the E isomer.
- 20Natural material was graciously provided by B. Shen and co-workers.
- 21Previous examples of phosphine-catalyzed E–Z equilibration:
- 21aC. Zhang, X. Lu, J. Org. Chem. 1995, 60, 2906–2908;
- 21bS. Ganguly, D. M. Roundhill, J. Chem. Soc. Chem. Commun. 1991, 639–640.
- 22This isomerization could also be effected by excessive exposure to silica gel or weak nucleophiles such as pyridine. The silica-promoted rearrangement also afforded a substantial quantity of migrastatin (2).
- 23For a discussion of the Curtin–Hammet principle, which is relevant to this discussion, see: J. I. Seeman, Pure Appl. Chem. 1987, 59, 1661–1672.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.