Efficient CuO-Nanoparticle-Catalyzed CS Cross-Coupling of Thiols with Iodobenzene†
Laxmidhar Rout
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India, Fax: (+91) 361-269-0762
Search for more papers by this authorTamal K. Sen
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India, Fax: (+91) 361-269-0762
Search for more papers by this authorTharmalingam Punniyamurthy Dr.
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India, Fax: (+91) 361-269-0762
Present Address: Chemistry Research Laboratory, University of Oxford, Oxford OX1 3TA, UK
Search for more papers by this authorLaxmidhar Rout
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India, Fax: (+91) 361-269-0762
Search for more papers by this authorTamal K. Sen
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India, Fax: (+91) 361-269-0762
Search for more papers by this authorTharmalingam Punniyamurthy Dr.
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India, Fax: (+91) 361-269-0762
Present Address: Chemistry Research Laboratory, University of Oxford, Oxford OX1 3TA, UK
Search for more papers by this authorThis research was supported by the Council of Scientific and Industrial Research, New Delhi (01(2002)/05/EMR-II) and the Department of Science and Technology, New Delhi (SR/S1/OC-09/2002).
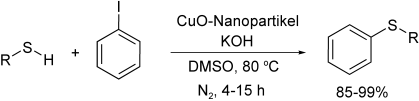
Graphical Abstract
Attraktiv und preiswert: Leicht erhältliche CuO-Nanopartikel kosten nicht nur weniger als andere Katalysatoren für die C-S-Kreuzkupplung, sie sind überdies schon bei moderaten Temperaturen und geringen Konzentrationen aktiv. Die Titelreaktion führt mit zahlreichen Alkyl- und Arylthiolen in hohen Ausbeuten zu den entsprechenden Sulfiden (siehe Schema).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701282_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. N. Jones, Comprehensive Organic Chemistry, Vol. 3 (Eds.: ), Pergamon, New York, 1979;
- 1bM. Tiecco, Synthesis 1988, 749;
- 1cC. M. Rayner, Contemp. Org. Synth. 1996, 3, 499;
- 1dC. P. Baird, C. M. Rayner, J. Chem. Soc. Perkin Trans. 1 1998, 1973;
- 1eD. J. Procter, J. Chem. Soc. Perkin Trans. 1 1999, 641;
- 1fD. J. Procter, J. Chem. Soc. Perkin Trans. 1 2001, 335;
- 1gC. G. Frost, P. Mendonca, J. Chem. Soc. Perkin Trans. 1 1998, 2615;
- 1hJ. Hassan, M. Sevignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 2002, 102, 1359;
- 1iI. P. Beletskaya, A. V. Cheprakov, Coord. Chem. Rev. 2004, 248, 2337;
- 1jJ.-P. Corbet, G. Mignani, Chem. Rev. 2006, 106, 2651;
- 1kF. Ullmann, Ber. Dtsch. Chem. Ges. 1903, 36, 2382;
- 1lJ. F. Hartwig, Angew. Chem. 1998, 110, 2154;
10.1002/(SICI)1521-3757(19980803)110:15<2154::AID-ANGE2154>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2046;10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 1mJ. P. Wolfe, S. Wagaw, J.-F. Marcoux, S. L. Buchwald, Acc. Chem. Res. 1998, 31, 805.
- 2
- 2aJ. Lindley, Tetrahedron 1984, 40, 1433;
- 2bT. Yamamoto, Y. Sekine, Can. J. Chem. 1984, 62, 1544;
- 2cR. J. S. Hickman, B. J. Christie, R. W. Guy, T. J. White, Aust. J. Chem. 1985, 38, 899;
- 2dA. Van Bierbeek, M. Gingras, Tetrahedron Lett. 1998, 39, 6283; DIBAL-H=diisobutylaluminum hydride.
- 3
- 3aT. Migita, T. Shimizu, Y. Asami, J. Shiobara, Y. Kato, M. Kosugi, Bull. Chem. Soc. Jpn. 1980, 53, 1385;
- 3bM. Kosugi, T. Ogata, M. Terada, H. Sano, T. Migita, Bull. Chem. Soc. Jpn. 1985, 58, 3657.
- 4
- 4aC. Mispelaere-Canivet, J.-F. Spindler, S. Perrio, P. Beslin, Tetrahedron 2005, 61, 5253;
- 4bT. Itoh, T. Mase, Org. Lett. 2004, 6, 4587;
- 4cM. A. Fernandez Rodrýguez, Q. Shen, J. F. Hartwig, J. Am. Chem. Soc. 2006, 128, 2180;
- 4dM. Murata, S. L. Buchwald, Tetrahedron 2004, 60, 7397;
- 4eU. Schopfer, A. Schlapbach, Tetrahedron 2001, 57, 3069;
- 4fG. Y. Li, Angew. Chem. 2001, 113, 1561;
Angew. Chem. Int. Ed. 2001, 40, 1513;
10.1002/1521-3773(20010417)40:8<1513::AID-ANIE1513>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 4gR. S. Barbiéri, C. R. Bellato, A. K. C. Dias, A. C. Massabni, Catal. Lett. 2006, 109, 171;
- 4hM. J. Dickens, J. P. Gilday, T. J. Mowlem, D. A. Widdowson, Tetrahedron 1991, 47, 8621;
- 4iT. Ishiyama, M. Mori, A. Suzuki, N. Miyaura, J. Organomet. Chem. 1996, 525, 225;
- 4jN. Zheng, J. C. McWilliams, F. J. Fleitz, J. D. Armstrong, R. P. Volante, J. Org. Chem. 1998, 63, 9606;
- 4kG. Mann, D. Baranano, J. F. Hartwig, A. L. Rheingold, I. A. Guzei, J. Am. Chem. Soc. 1998, 120, 9205.
- 5
- 5aH. J. Cristau, B. Chabaud, A. Chene, H. Christol, Synthesis 1981, 892;
- 5bC. Millois, P. Diaz, Org. Lett. 2000, 2, 1705;
- 5cV. Percec, J.-Y. Bae, D. H. Hill, J. Org. Chem. 1995, 60, 6895;
- 5dK. Takagi, Chem. Lett. 1987, 2221.
- 6
- 6aC. G. Bates, R. K. Gujadhur, D. Venkataraman, Org. Lett. 2002, 4, 2803;
- 6bF. Y. Kwong, S. L. Buchwald, Org. Lett. 2002, 4, 3517;
- 6cY.-J. Wu, H. He, Synlett 2003, 1789;
- 6dC. G. Bates, P. Saejueng, M. Q. Doherty, D. Venkataraman, Org. Lett. 2004, 6, 5005;
- 6eW. Deng, Y. Zou, Y.-F. Wang, L. Liu, Q.-X. Guo, Synlett 2004, 1254;
- 6fC. Palomo, M. Oiarbide, R. Lopez, E. Gomez-Bengoa, Tetrahedron Lett. 2000, 41, 1283;
- 6gC. Savarin, J. Srogl, L. S. Liebeskind, Org. Lett. 2002, 4, 4309;
- 6hP. S. Herradura, K. A. Pendola, R. K. Guy, Org. Lett. 2000, 2, 2019;
- 6iY.-J. Chen, H.-H. Chen, Org. Lett. 2006, 8, 5609;
- 6jD. Zhu, L. Xu, F. Wu, B. S. Wan, Tetrahedron Lett. 2006, 47, 5781;
- 6kS. V. Ley, A. W. Thomas, Angew. Chem. 2003, 115, 5558;
10.1002/ange.200300594 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5400.
- 7Y.-C. Wong, T. T. Jayanth, C.-H. Cheng, Org. Lett. 2006, 8, 5613.
- 8
- 8aT. Punniyamurthy, S. Velusamy, J. Iqbal, Chem. Rev. 2005, 105, 2329;
- 8bL. Rout, T. Punniyamurthy, Adv. Synth. Catal. 2005, 347, 1958;
- 8cS. Velusamy, M. Ahamed, T. Punniyamurthy, Org. Lett. 2004, 6, 4821;
- 8dC. L. Hill, Nature 1999, 401, 436;
- 8eR. A. Sheldon, Pure Appl. Chem. 2000, 72, 1233;
- 8fH.-U. Blaser, A. Indolese, F. Naud, U. Nettekoven, A. Schnyder, Adv. Synth. Catal. 2004, 346, 1583.
- 9
- 9aB. F. G. Johnson, Coord. Chem. Rev. 1999, 190–192, 1269;
- 9bL. N. Lewis, Chem. Rev. 1993, 93, 2693;
- 9cM. T. Reetz, E. Westermann, Angew. Chem. 2000, 112, 170;
10.1002/(SICI)1521-3757(20000103)112:1<170::AID-ANGE170>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2000, 39, 165;10.1002/(SICI)1521-3773(20000103)39:1<165::AID-ANIE165>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 9dY. Li, X. M. Hong, D. M. Collard, M. A. El-Sayed, Org. Lett. 2000, 2, 2385;
- 9eK. S. Weddle, J. D. Aiken, R. G. Finke, J. Am. Chem. Soc. 1998, 120, 5653;
- 9fM. Zhao, R. M. Crooks, Angew. Chem. 1999, 111, 375;
Angew. Chem. Int. Ed. 1999, 38, 364.
10.1002/(SICI)1521-3773(19990201)38:3<364::AID-ANIE364>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.