Asymmetric Organocatalytic α-Arylation of Aldehydes†
José Alemán Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorSilvia Cabrera Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorEddy Maerten Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorJacob Overgaard Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorKarl Anker Jørgensen Prof. Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorJosé Alemán Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorSilvia Cabrera Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorEddy Maerten Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorJacob Overgaard Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorKarl Anker Jørgensen Prof. Dr.
Danish National Research Foundation: Center for Catalysis Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorThis work was made possible by a grant from the Danish National Research Foundation. J.A. and S.C. thank the Ministerio de Educación y Ciencia of Spain for a postdoctoral fellowship.
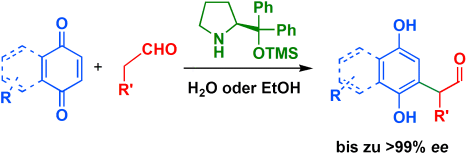
Graphical Abstract
Chinone als Helfer: Die organokatalytische enantioselektive α-Arylierung von Aldehyden mit Chinonen als dem aromatischen Partner verläuft in H2O oder EtOH/H2O-Mischungen sehr effizient. Die optisch aktiven α-arylierten Aldehyde werden in hohen Ausbeuten und mit ausgezeichneten Enantioselektivitäten gebildet (siehe Schema; TMS: Trimethylsilyl).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701207_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1See, for example:
- 1aJ. Ahman, J. P. Wolfe, M. V. Troutman, M. Palucki, S. L. Buchwald, J. Am. Chem. Soc. 1998, 120, 1918;
- 1bT. Hamada, A. Chieffi, J. Ahman, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1261;
- 1cD. J. Spielvogel, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 3500, and references therein;
- 1dG. C. Lloyd-Jones, Angew. Chem. 2002, 114, 995;
10.1002/1521-3757(20020315)114:6<995::AID-ANGE995>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 953;10.1002/1521-3773(20020315)41:6<953::AID-ANIE953>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 1eD. A. Culkin, J. F. Hartwig, Acc. Chem. Res. 2003, 36, 234, and references therein.
- 2For recent reviews on organocatalysis, see, for example:
- 2aP. L. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138;
- 2bAcc. Chem. Res. 2004, 37(8), special issue on organocatalysis;
- 2cA. Berkessel, H. Gröger, Asymmetric Organocatalysis, VCH, Weinheim, 2005;
- 2dJ. Seayad, B. List, Org. Biomol. Chem. 2005, 3, 719;
- 2eB. List, J.-W. Yang, Science 2006, 313, 1584;
- 2fM. J. Gaunt, C. C. C. Johansson, A. McNally, N. C. Vo, Drug Discovery Today 2007, 12, 8;
- 2gB. List, Chem. Commun. 2006, 819;
- 2hP. I. Dalko, Enantioselective Organocatalysis, Wiley-VCH, Weinheim, 2007.
10.1002/9783527610945 Google Scholar
- 3See, for example:
- 3aM. Marigo, K. A. Jørgensen, Chem. Commun. 2006, 2001;
- 3bG. Guillena, D. J. Ramón, Tetrahedron: Asymmetry 2006, 17, 1465;
- 3cN. Mase, K. Watanabe, H. Yoda, K. Tanabe, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 4966;
- 3dS. Samanta, C.-G. Zhao, J. Am. Chem. Soc. 2006, 128, 7442;
- 3eY. Hayashi, T. Sumiya, J. Takahashi, H. Gotoh, T. Urushima, M. Shoji, Angew. Chem. 2006, 118, 972; Angew. Chem. Int. Ed. 2006, 45, 958;
- 3fT. Kano, M. Ueda, J. Takai, K. Maruoka, J. Am. Chem. Soc. 2006, 128, 6046.
- 4See e.g.:
- 4aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 4bS. G. Ouellet, J. B. Tuttle, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 32;
- 4cS. Mayer, B. List, Angew. Chem. 2006, 118, 4299; Angew. Chem. Int. Ed. 2006, 45, 4193;
- 4dM. Marigo, M. T. Schulte, J. Franzén, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 15710;
- 4eS. Brandau, A. Landa, J. Franzén, M. Marigo, K. A. Jørgensen, Angew. Chem. 2006, 118, 4411;
10.1002/ange.200601025 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4305;
- 4fH. Gotoh, R. Masui, H. Ogino, M. Shoji, Y. Hayashi, Angew. Chem. 2006, 118, 7007;
10.1002/ange.200602925 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6853;
- 4gW. Wang, H. Li, J. Wang, L. Zu, J. Am. Chem. Soc. 2006, 128, 10354;
- 4hS. Brandau, E. Maerten, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 14986;
- 4iY. K. Chen, M. Yoshida, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 9328;
- 4jH. Sundén, I. Ibrahem, G.-L. Zhao, L. Eriksson, A. Córdova, Chem. Eur. J. 2007, 13, 574;
- 4kfor a general review of β-funtionalization, see: S. B. Tsogoeva, Eur. J. Org. Chem. 2007, 1701.
- 5S. Bertelsen, M. Marigo, S. Brandes, P. Dinér, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 12973.
- 6
- 6a The Chemistry of the Quinoid Compounds (Eds: ), Wiley, New York, 1988;
- 6bfor a review of quinone chemistry, see: A. Kutyrev, Tetrahedron 1991, 47, 8043;
- 6csee also: M. E. Kuehne, J. Am. Chem. Soc. 1962, 84, 837.
- 7See, for example:
- 7aJ. Franzén, M. Marigo, D. Fielenbach, T. C. Wabnitz, A. Kjærsgaard, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 18296;
- 7bC. Palomo, A. Mielgo, Angew. Chem. 2006, 118, 8042; Angew. Chem. Int. Ed. 2006, 45, 7876;
- 7cG.-L. Zhao, A. Córdova, Tetrahedron Lett. 2006, 47, 7417;
- 7dT. Govender, L. Hojabri, F. M. Moghaddam, P. I. Arvidsson, Tetrahedron: Asymmetry 2006, 17, 1763;
- 7eD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 7fI. Ibrahem, G.-L. Zhao, H. Sundén, A. Córdova, Tetrahedron Lett. 2006, 47, 4659;
- 7gY. Chi, S. H. Gellman, J. Am. Chem. Soc. 2006, 128, 6804;
- 7hS. S. Chow, M. Nevalainen, C. A. Evans, C. W. Johannes, Tetrahedron Lett. 2007, 48, 277;
- 7isee also Refs [4f,g].
- 8CCDC 639134 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9For a recent discussion about water in organocatalytic processes, see: D. G. Blackmond, A. Armstrong, V. Coombe, A. Wells, Angew. Chem. 2007, 119, 3872;
10.1002/ange.200604952 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3798, and references therein.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.