Enantioselective Synthesis of Oasomycin A, Part I: Synthesis of the C1–C12 and C13–C28 Subunits†
David A. Evans Prof.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorPavel Nagorny
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorKenneth J. McRae Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorDominic J. Reynolds Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorLouis-Sebastian Sonntag Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorFilisaty Vounatsos Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorRisheng Xu
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorDavid A. Evans Prof.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorPavel Nagorny
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorKenneth J. McRae Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorDominic J. Reynolds Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorLouis-Sebastian Sonntag Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorFilisaty Vounatsos Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorRisheng Xu
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617-495-1460
Search for more papers by this authorFinancial support has been provided by the National Institutes of Health (GM-33327-19), the Merck Research Laboratories, Amgen, and Eli Lilly. A postdoctoral fellowship was provided to L.-S.S. by the Deutscher Akademischer Austauschdienst and the Novartis Foundation, and to D.J.R. by the Glaxo Foundation.
Graphical Abstract
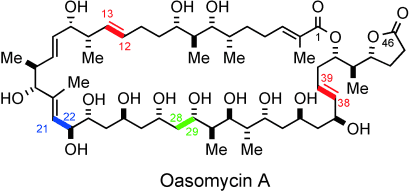
Vom Einzelnen zum Ganzen: Die Totalsynthese des natürlichen Makrolids Oasomycin A wurde abgeschlossen. Zu den zentralen Fragmentverknüpfungen gehören eine anti-Felkin-selektive Aldoladdition (grün), Kociensky-Julia-Olefinierungen (rot) und eine konkurrierende Weinreb-Amid-Acylierung (blau). Die Nützlichkeit von 4,5-Diphenyloxazol als Carboxy-Ersatz und die zu einem späten Zeitpunkt durchgeführte Makrolactonisierung, die den 42-gliedrigen Makrocyclus von Oasomycin A liefert, werden ebenfalls beschrieben.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z603653_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Grabley, G. Kretzschmar, M. Mayer, S. Philipps, R. Thiericke, J. Wink, A. Zeeck, Liebigs Ann. Chem. 1993, 5, 573–579;
10.1002/jlac.199319930193 Google Scholar
- 1bM. Mayer, R. Thiericke, J. Chem. Soc. Perkin Trans. 1 1993, 21, 2525–2531.
- 2For the stereochemical assignment of oasomycin A, see: Y. Kobayashi, S.-H. Tan, Y. Kishi, J. Am. Chem. Soc. 2001, 123, 2076–2078, and references therein.
- 3For the preparation of 1, see:
- 3aD. A. Evans, M. D. Ennis, T. Le, N. Mandel, G. Mandel, J. Am. Chem. Soc. 1984, 106, 1154–1156;
- 3bD. A. Evans, J. R. Gage, Org. Synth. 1989, 68, 83–91.
- 4T. Ishikawa, S. Ikeda, M. Ibe, S. Saito, Tetrahedron 1998, 54, 5869–5882.
- 5A. M. P. Koskien, K. Karisalmi, Chem. Soc. Rev. 2005, 34, 677–690.
- 6The relative stereochemistry of the reduction was proven by converting the product resulting from reduction of 3 into an acetonide. The 13C NMR signals (δ=97.9, 29.9, and 19.4 ppm) for the acetonide moiety confirmed that the aforementioned reduction proceeded to afford the syn diastereomer.
- 6aS. D. Rychnovsky, D. J. Skalitzky, Tetrahedron Lett. 1990, 31, 945–948;
- 6bS. D. Rychnovsky, B. Rogers, G. Yang, J. Org. Chem. 1997, 62, 3511–3515;
- 6cD. A. Evans, D. L. Rieger, J. R. Gage, Tetrahedron Lett. 1990, 31, 7099–7100.
- 7T. Oishi, T. Nakata, Acc. Chem. Res. 1984, 17, 338–344.
- 8For the preparation of phosphonate 5, see:
- 8aG. Pattenden, B. C. L. Weedon, J. Chem. Soc. C 1968, 1984–1997;
- 8bT. Kitara, A. Horiguchi, K. Mori, Tetrahedron 1988, 44, 4713–4720.
- 9Attempts to eliminate the inseparable side products were unsuccessful under a variety of different conditions. The Lindlar catalyst in ethyl acetate provided the best selectivity (5:1:1 favoring reduction of the Δ4 olefin).
- 10R. H. Crabtree, M. W. Davis, J. Org. Chem. 1986, 51, 2655–2661.
- 11The mixture was separated by medium-pressure chromatography (MPLC).
- 12The acidic nature of [(NH4)6Mo7O24] resulted in partial cleavage of the secondary TES group of 3 before the sulfoxide oxidation was complete (8–10 %). The corresponding desilylated product could be recovered and reprotected (TESOTf, lutidine).
- 13H. S. Schultz, H. B. Freyermuth, S. R. Buc, J. Org. Chem. 1963, 28, 1140–1142.
- 14R. Baker, J. L. Castro, J. Chem. Soc. Perkin Trans. 1 1990, 47–65, and references therein.
- 15For the synthesis of enantiomer 22, see:
- 15aS. D. Rychnovsky, J. Org. Chem. 1989, 54, 4982–4984;
- 15bJ. A. Christopher, P. J. Kocienski, A. Kuhl, R. Bell, Synlett 2000, 463–466;
- 15cM. J. Remuiñán, G. Pattenden, Tetrahedron Lett. 2000, 41, 7367–7371.
- 16J. R. Parikh, W. von Doering, J. Am. Chem. Soc. 1967, 89, 5505–5507.
- 17D. A. Evans, B. D. Allison, M. G. Yang, C. E. Masse, J. Am. Chem. Soc. 2001, 123, 10840–10852. The stereochemistry of the major allylation product was proven by comparing the spectroscopic characteristics of 22 to analogous compound 22 a, the stereochemistry of which was confirmed by X-ray crystallography.
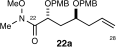
- 18B. S. Bal, W. E. Childers, H. W. Pinnick, Tetrahedron 1981, 37, 2091–2096.
- 19J. M. Williams, R. B. Jobson, N. Yasuda, G. Marchesini, U.-H. Dolling, E. J. Grabowski, Tetrahedron Lett. 1995, 36, 5461–5464.
- 20For examples of acetoxysuccinic anhydride reactivity that illustrate the mentioned effect, see: D. J. Hart, T. K. Yang, J. Org. Chem. 1985, 50, 235–242; S. Gogoi, N. P. Argade, Tetrahedron 2006, 62, 2999–3003, and references therein.
- 21Both the presence of THF and transmetalation of 16 b to 16 c were essential for this reaction to run to completion. Thus, the acylations of 16 b and 16 c in the absence of THF proceeded in 45 % and 66 % yield respectively.
- 22Y. Oikawa, T. Tanaka, K. Horita, T. Yoshioka, O. Yonemitsu, Tetrahedron Lett. 1984, 25, 5393–5396.
- 23
- 23aD. A. Evans, P. Nagorny, K. J. McRae, D. J. Reynolds, L.-S. Sonntag, F. Vounatsos, R. Xu, Angew. Chem. 2007, 119, 547–550; Angew. Chem. Int. Ed. 2007, 46, 541–544;
- 23bD. A. Evans, P. Nagorny, K. J. McRae, L.-S. Sonntag, D. J. Reynolds, F. Vounatsos, Angew. Chem. 2007, 119, 551–554; Angew. Chem. Int. Ed. 2007, 46, 545–548.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.