Aqueous FeIVO: Spectroscopic Identification and Oxo-Group Exchange†
Correction(s) for this article
-
Aqueous FeIVO: Spectroscopic Identification and Oxo-Group Exchange
- Oleg Pestovsky Dr.,
- Sebastian Stoian,
- Emile L. Bominaar Prof. Dr.,
- Xiaopeng Shan Dr.,
- Eckard Münck Prof. Dr.,
- Lawrence Que Prof. Dr.,
- Andreja Bakac Prof. Dr.,
- Volume 118Issue 3Angewandte Chemie
- pages: 348-348
- First Published online: January 5, 2006
Oleg Pestovsky Dr.
Ames Laboratory, Iowa State University, Ames, IA 50011, USA, Fax: (+1) 515-294-5233
Search for more papers by this authorSebastian Stoian
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 12513, USA, Fax: (+1) 412-268-1061
Search for more papers by this authorEmile L. Bominaar Prof. Dr.
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 12513, USA, Fax: (+1) 412-268-1061
Search for more papers by this authorXiaopeng Shan Dr.
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-624-7029
Search for more papers by this authorEckard Münck Prof. Dr.
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 12513, USA, Fax: (+1) 412-268-1061
Search for more papers by this authorLawrence Que Jr. Prof. Dr.
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-624-7029
Search for more papers by this authorAndreja Bakac Prof. Dr.
Ames Laboratory, Iowa State University, Ames, IA 50011, USA, Fax: (+1) 515-294-5233
Search for more papers by this authorOleg Pestovsky Dr.
Ames Laboratory, Iowa State University, Ames, IA 50011, USA, Fax: (+1) 515-294-5233
Search for more papers by this authorSebastian Stoian
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 12513, USA, Fax: (+1) 412-268-1061
Search for more papers by this authorEmile L. Bominaar Prof. Dr.
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 12513, USA, Fax: (+1) 412-268-1061
Search for more papers by this authorXiaopeng Shan Dr.
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-624-7029
Search for more papers by this authorEckard Münck Prof. Dr.
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 12513, USA, Fax: (+1) 412-268-1061
Search for more papers by this authorLawrence Que Jr. Prof. Dr.
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-624-7029
Search for more papers by this authorAndreja Bakac Prof. Dr.
Ames Laboratory, Iowa State University, Ames, IA 50011, USA, Fax: (+1) 515-294-5233
Search for more papers by this authorSupported under DOE Contract No. W-7405-ENG-82 (A.B.) and NIH grants EB001475 (E.M.) and GM-38767 (L.Q.)
Graphical Abstract
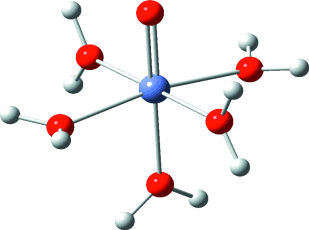
Eine Kombination von Techniken (Mößbauer- und Röntgenabsorptionsspektroskopie, H218O-Austauschexperimente und DFT-Rechnungen) ergab, dass bei der Reaktion von [Fe(H2O)6]2+ mit Ozon [(H2O)5FeIVO]2+ gebildet wird (siehe die berechnete Struktur). Die Oxidation ausgewählter Substrate mit [(H2O)5FeIVO]2+ lieferte Produkte, die sich von denen unterscheiden, die bei der Fenton-Reaktion oder bei Umsetzungen mit HO.-Radikalen entstehen.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z502686_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. J. H. Fenton, J. Chem. Soc. 1894, 65, 899.
- 2F. Haber, J. Weiss, Proc. R. Soc. London 1930, 147, 332.
10.1098/rspa.1934.0221 Google Scholar
- 3W. C. Bray, M. H. Gorin, J. Am. Chem. Soc. 1932, 54, 2124.
- 4W. H. Koppenol, Free Radical Biol. Med. 1993, 15, 645.
- 5M. Masarwa, H. Cohen, D. Meyerstein, D. L. Hickman, A. Bakac, J. H. Espenson, J. Am. Chem. Soc. 1988, 110, 4293.
- 6C. Walling, Acc. Chem. Res. 1998, 31, 155.
- 7C. Walling, Acc. Chem. Res. 1975, 8, 125.
- 8F. Buda, B. Ensing, M. C. M. Gribnau, E. J. Baerends, Chem. Eur. J. 2003, 9, 3436.
- 9T. J. Conocchioli, E. J. Hamilton, Jr.,N. Sutin, J. Am. Chem. Soc. 1965, 87, 926.
- 10T. Logager, J. Holcman, K. Sehested, T. Pedersen, Inorg. Chem. 1992, 31, 3523.
- 11O. Pestovsky, A. Bakac, J. Am. Chem. Soc. 2004, 126, 13757.
- 12F. Jacobsen, J. Holcman, K. Sehested, Int. J. Chem. Kinet. 1998, 30, 215.
- 13J. E. Knudsen, J. Phys. Chem. Solids 1980, 41, 545.
- 14J.-U. Rohde, J.-H. In, M. H. Lim, W. W. Brennessel, M. R. Bukowski, A. Stubna, E. Münck, W. Nam, L. Que, Jr., Science 2003, 299, 1037.
- 15M. H. Lim, J.-U. Rohde, A. Stubna, M. R. Bukowski, M. Costas, R. Y. N. Ho, E. Münck, W. Nam, L. Que, Jr., Proc. Natl. Acad. Sci. USA 2003, 100, 3665.
- 16C. Krebs, J. C. Price, J. Baldwin, L. Saleh, M. T. Green, J. M. Bollinger, Jr., Inorg. Chem. 2005, 44, 742.
- 17J.-U. Rohde, S. Torelli, X. Shan, M. H. Lim, E. J. Klinker, J. Kaizer, K. Chen, W. Nam, L. Que, Jr., J. Am. Chem. Soc. 2004, 126, 16750.
- 18P. J. Riggs-Gelasco, J. C. Price, R. B. Guyer, J. H. Brehm, E. W. Barr, J. M. Bollinger, Jr.,C. Krebs, J. Am. Chem. Soc. 2004, 126, 8108.
- 19A. Decker, E. I. Solomon, Angew. Chem. 2005, 44, 2292; Angew. Chem. Int. Ed. 2005, 44, 2252.
- 20T. E. Westre, P. Kennepohl, J. G. DeWitt, B. Hedman, K. O. Hodgson, E. I. Solomon, J. Am. Chem. Soc. 1997, 119, 6297.
- 21B. A. Sommer, D. W. Margerum, Inorg. Chem. 1970, 9, 2517.
- 22B. C. Gilbert, R. O. C. Norman, R. C. Sealy, J. Chem. Soc. Perkin Trans. 2 1975, 303.
- 23M. K. Eberhardt, R. Colina, J. Org. Chem. 1988, 53.
- 24Our data, however, do not address the question of whether {FeIVO} species are formed in the reaction of iron(II) salts with H2O2 in acetonitrile, as first discussed in the seminal papers: J. T. Groves, M. Van Der Puy, J. Am. Chem. Soc. 1974, 96, 5274; J. T. Groves, G. A. McClusky, J. Am. Chem. Soc. 1976, 98, 859.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.