Recyclable Palladium Catalyst for Highly Selective α Alkylation of Ketones with Alcohols†
Min Serk Kwon
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorNamdu Kim
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorSeong Hyeok Seo
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorIn Soo Park
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorRavi Kumar Cheedrala
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorJaiwook Park Prof.
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorMin Serk Kwon
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorNamdu Kim
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorSeong Hyeok Seo
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorIn Soo Park
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorRavi Kumar Cheedrala
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorJaiwook Park Prof.
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology (POSTECH), San 31 Hyoja Dong, Pohang 790-784, Korea, Fax: (+82) 54-279-3399
Search for more papers by this authorThis work was supported by the SRC/ERC program of MOST/KOSEF (R11-2000-070-05003-0).
Graphical Abstract
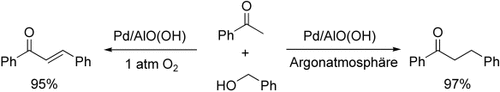
Luftbeständig und wiederverwendbar ist ein Heterogenkatalysator aus Palladium-Nanopartikeln in einer Aluminiumhydroxidmatrix. Die hoch selektive α-Alkylierung von aliphatischen und aromatischen Ketonen mit primären Alkoholen führte in einer O2-Atmosphäre zu Enonen und in einer Argonatmosphäre zu Ketonen (siehe Schema).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z502422_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. Caine in Comprehensive Organic Synthesis, Vol. 3 (Eds.: ), Pergamon, Oxford, 1991, pp. 1–63;
- 1bS. Carrettin, J. Guzman, A. Corma, Angew. Chem. 2005, 117, 2282–2285;
10.1002/ange.200462560 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2242–2245;
- 1c Modern Carbonyl Chemistry (Ed.: ), Wiley-VCH, Weinheim, 2000.
- 2
- 2aC. S. Cho, B. T. Kim, M. J. Lee, T.-J. Kim, S. C. Shim, Angew. Chem. 2001, 113, 984–986;
Angew. Chem. Int. Ed. 2001, 40, 958–960;
10.1002/1521-3773(20010302)40:5<958::AID-ANIE958>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 2bC. S. Cho, B. T. Kim, T.-J. Kim, S. C. Shim, J. Org. Chem. 2001, 66, 9020–9022;
- 2cC. S. Cho, B. T. Kim, T.-J. Kim, S. C. Shim, Tetrahedron Lett. 2002, 43, 7987–7989;
- 2dC. S. Cho, B. T. Kim, H.-S. Kim, T.-J. Kim, S. C. Shim, Organometallics 2003, 22, 3609–3610;
- 2eK. Taguchi, H. Nakagawa, T. Hirabayashi, S. Sakaguchi, Y. Ishii, J. Am. Chem. Soc. 2004, 126, 72–73;
- 2fR. Martínez, G.-J. Brand, D.-J. Ramón, M. Yus, Tetrahedron Lett. 2005, 46, 3683–3686.
- 3K. Motokura, D. Nishimura, K. Mori, T. Mizugaki, K. Ebitani, K. Kaneda, J. Am. Chem. Soc. 2004, 126, 5662–5663.
- 4M. S. Kwon, N. Kim, C. M. Park, J. S. Lee, K. Y. Kang, J. Park, Org. Lett. 2005, 7, 1077–1079.
- 5The coupling reaction was completed in 16 h (10 h) when one equivalent (2 equiv) of K3PO4 was used.
- 6The major product is the corresponding alcohol when [Ru(dmso)4]Cl2 is used as the catalyst; see ref. [2f].
- 7Pregnenolone is a main precursor of steroid hormones: K. Tsutsui, H. Sakamoto, K. Ukena, J. Steroid Biochem. Mol. Biol. 2003, 85, 311–321.
- 8According to 1H NMR spectroscopic analysis, no diastereomer of the major coupling product was formed in significant yield.
- 9Palladium was not detected in the filtrate with inductively coupled plasma (ICP) analysis.
- 10The NMR spectroscopic data for the coupling products shown in Table 3 and the experimental procedures for the preparation of 1, E-1,3-diphenyl-2-propen-1-one, and 21-benzylpregnenolone are contained in the Supporting Information.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.