Reactions of Gold(III) Oxo Complexes with Cyclic Alkenes†
Maria A. Cinellu Prof.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorGiovanni Minghetti Prof.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorFabio Cocco
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorSergio Stoccoro Dr.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorAntonio Zucca Dr.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorMario Manassero Prof.
Dipartimento di Chimica Strutturale e Stereochimica Inorganica, Università di Milano, Centro CNR via Venezian 21, 20133 Milano, Italy
Search for more papers by this authorMaria A. Cinellu Prof.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorGiovanni Minghetti Prof.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
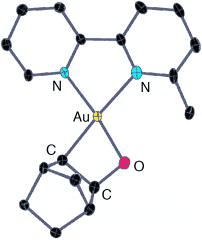
Search for more papers by this authorFabio Cocco
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorSergio Stoccoro Dr.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorAntonio Zucca Dr.
Dipartimento di Chimica, Università di Sassari via Vienna 2, 07100 Sassari, Italy, Fax: (+39) 079-229-559
Search for more papers by this authorMario Manassero Prof.
Dipartimento di Chimica Strutturale e Stereochimica Inorganica, Università di Milano, Centro CNR via Venezian 21, 20133 Milano, Italy
Search for more papers by this authorSupport from the University of Sassari (60 %) is gratefully acknowledged. We thank Mrs. E. Azara (ICB, CNR, Sassari) and Dr. A. Furesi (PMP, USSL, Sassari) for performing GC-MS and LC-MS analyses. Thanks are also due to Dr. G. A. Chelucci (Dipartimento di Chimica, Università di Sassari) for support in the organic syntheses and simulation of NMR spectra.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z501754_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. de Bruin, P. H. M. Budzelaar, A. W. Gal, Angew. Chem. 2004, 116, 4236–4251; Angew. Chem. Int. Ed. 2004, 43, 4142–4156.
- 2
- 2aK. B. Sharpless, A. Y. Teranishi, J.-E. Bäckvall, J. Am. Chem. Soc. 1977, 99, 3120–3128;
- 2bK. A. Jørgensen, B. Schiøtt, Chem. Rev. 1990, 90, 1483–1506.
- 3S. Linic, H. Piao, K. Adib, M. A. Barteau, Angew. Chem. 2004, 116, 2978–2981;
10.1002/ange.200353584 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2918–2921.
- 4E. Szuromi, H. Shan, P. R. Sharp, J. Am. Chem. Soc. 2003, 125, 10522–10523.
- 5T. Hosokawa, M. Takano, S.-I. Murahashi, J. Am. Chem. Soc. 1996, 118, 3990–3991.
- 6
- 6aV. W. Day, W. G. Klemperer, S. P. Lockledge, D. J. Main, J. Am. Chem. Soc. 1990, 112, 2031–2033;
- 6bB. de Bruin, M. J. Boerakker, J. J. J. M. Donners, B. E. C. Christiaans, P. P. J. Schlebos, R. de Gelder, J. M. M. Smits, A. L. Spek, Angew. Chem. 1997, 109, 2153–2157; Angew. Chem. Int. Ed. Engl. 1997, 36, 2064–2067;
- 6cB. de Bruin, J. A. Brands, J. J. J. M. Donners, M. P. J. Donners, R. de Gelder, J. M. M. Smits, A. W. Gal, A. L. Spek, Chem. Eur. J. 1999, 5, 2921–2936;
10.1002/(SICI)1521-3765(19991001)5:10<2921::AID-CHEM2921>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 6dT. C. Flood, M. Iimura, J. M. Perotti, A. L. Rheingold, T. E. Concolino, Chem. Commun. 2000, 1681–1682;
- 6eB. de Bruin, M. J. Boerakker, J. A. W. Verhagen, R. de Gelder, A. W. Gal, Chem. Eur. J. 2000, 6, 298–312;
10.1002/(SICI)1521-3765(20000117)6:2<298::AID-CHEM298>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 6fB. de Bruin, J. A. W. Verhagen, C. H. J. Schouten, A. W. Gal, D. Feichtinger, D. A. Plattner, Chem. Eur. J. 2001, 7, 416–422.
10.1002/1521-3765(20010119)7:2<416::AID-CHEM416>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 7
- 7aM. Haruta, Gold Bull. 2004, 37, 27–36;
- 7bG. C. Bond, D. T. Thompson, Gold Bull. 2000, 33, 41–50;
- 7cH. H. Kung, M. C. Kung, C. K. Costello, J. Catal. 2003, 216, 425–432;
- 7dJ. R. Monnier, Appl. Catal. A 2001, 221, 73–91.
- 8A. K. Sinha, S. Seelan, S. Tsubota, M. Haruta, Angew. Chem. 2004, 116, 1572–1574;
10.1002/ange.200352900 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1546–1548.
- 9M. A. Cinellu, G. Minghetti, M. V. Pinna, S. Stoccoro, A. Zucca, M. Manassero, M. Sansoni, J. Chem. Soc. Dalton Trans. 1998, 1735–1741.
- 10D. Rais, Dissertation, Università di Sassari, 1998.
- 11M. A. Cinellu, G. Minghetti, S. Stoccoro, A. Zucca, M. Manassero, Chem. Commun. 2004, 1618–1619.
- 12Analogous alkene complexes are likewise obtained with other 1-alkenes, whereas no reaction occurs with linear internal alkenes; a small amount of an oxygenated gold species is also detected by 1H NMR spectroscopy and FAB-MS of the α-methylstyrene derivative. Oxygenated organic products were characterized. Styrene derivatives: phenylacetaldehyde (main product) and benzaldehyde, reaction in MeCN; phenylacetaldehyde dimethyl acetal, reaction in MeCN/MeOH; styrene glycol, reaction in MeCN/H2O. α-Methylstyrene derivatives: acetophenone, reaction in MeCN; 2-phenyl-1,2-propanediol, reaction in MeCN/H2O; this diol converts almost completely into methylbenzylketone after a prolonged reaction time.
- 13Part of this work was presented in oral form: M. A. Cinellu, F. Cocco, G. Minghetti, S. Stoccoro, A. Zucca, M. Manassero, III Euchem Conference on Nitrogen Ligands in Organometallic Chemistry and Homogeneous Catalysis, Camerino, Italy, September 8–12, 2004, O3.
- 14For selected reviews on homogeneous catalysis by gold, see:
- 14aA. Hoffmann-Röder, N. Krause, Org. Biomol. Chem., 2005, 3, 387–391;
- 14bA. S. K. Hashmi, Gold Bull. 2004, 37, 51–65;
- 14cA. S. K. Hashmi, Gold Bull. 2003, 36, 3–9;
- 14dG. Dyker, Angew. Chem. 2000, 112, 4407–4409;
10.1002/1521-3757(20001201)112:23<4407::AID-ANGE4407>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4237–4239; selected examples of alkyne transformations:10.1002/1521-3773(20001201)39:23<4237::AID-ANIE4237>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 14eY. Fukuda, K. Utimoto, J. Org. Chem. 1991, 56, 3729–3731;
- 14fJ. H. Teles, S. Brode, M. Chabanas, Angew. Chem. 1998, 110, 1475–1478;
10.1002/(SICI)1521-3757(19980518)110:10<1475::AID-ANGE1475>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1415–1418;10.1002/(SICI)1521-3773(19980605)37:10<1415::AID-ANIE1415>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 14gA. S. K. Hashmi, T. M. Frost, J. W. Bats, Org. Lett. 2001, 3, 3769–3771;
- 14hE. Mizushima, K. Saro, T. Hayashi, M. Tanaka, Angew. Chem. 2002, 114, 4745–4747;
10.1002/1521-3757(20021202)114:23<4745::AID-ANGE4745>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4563–4565;10.1002/1521-3773(20021202)41:23<4563::AID-ANIE4563>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 14iR. Casado, M. Contel, M. Laguna, P. Romero, S. Sanz, J. Am. Chem. Soc. 2003, 125, 11925–11935;
- 14jT. Yao, X. Zhang, R. C. Larock, J. Am. Chem. Soc. 2004, 126, 11164–11165;
- 14kA. S. K. Hashmi, J. P. Weyrauch, W. Frey, J. W. Bats, Org. Lett. 2004, 6, 4391–4394;
- 14lV. Mamane, T. Gress, H. Krause, A. Fürstner, J. Am. Chem. Soc. 2004, 126, 8654–8655;
- 14mA. S. K. Hashmi, P. Sinha, Adv. Synth. Catal. 2004, 346, 432–438;
- 14nA. S. K. Hashmi, M. Rudolf, J. P. Weyrauch, M. Wölfle, W. Frey, J. W. Bats, Angew. Chem. 2005, 117, 2858–2861; Angew. Chem. Int. Ed. 2005, 44, 2798–2801; selected examples of alkene transformations:
- 14oA. S. K. Hashmi, L. Schwarz, J.-H. Choi, T. M. Frost, Angew. Chem. 2000, 112, 2382–2385;
10.1002/1521-3757(20000703)112:13<2382::AID-ANGE2382>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2285–2288;10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 14pS. Kobayashi, K. Kakumoto, M. Sugiura, Org. Lett. 2002, 4, 1319–1322;
- 14qC.-G. Yang, C. He, J. Am. Chem. Soc. 2005, 127, 6966–6967.
- 15For full experimental data on the synthesis, spectroscopy, and elemental analysis of complexes 2 a–2 c, 3 a–3 d, 4 a–4 c and for spectroscopic characterization of organic compounds 6–11 see Supporting Information.
- 16Crystal data for 3 a-PF6: C18H20AuF6N2P, Mr=606.30 g mol−1, orthorhombic, space group Pna21 (no. 33), a=16.480(4), b=16.662(3), c=6.911(2) Å, V=1897.7(7) Å3.
- 17Crystal data for 4 a-PF6: C18H20AuF6N2OP, Mr=622.30 g mol−1, triclinic, space group P
 (no. 2), a=6.466(1), b=8.822(1), c=17.548(1) Å, α=103.45(1), β=90.38(1), γ=100.97(1)°, V=954.4(2) Å3, Z=2, ρcalcd=2.165 g cm−3, T=150 K, μ(MoKα)=78.4 cm−1, F(000)=596. Reflections measured 19 839, independent 5795 with Rint=0.043. Final R2 (F 2, all reflections)=0.113, wR2=0.165, conventional R1=0.052 for 262 variables. Bruker SMART CCD area detector, MoKα radiation (λ=0.71073 Å), ω-scan mode, θmin=3°, θmax=26°. Structure solved by direct methods and refined by full-matrix least squares. The program used to refine the structure was Personal SDP. CCDC-272640 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
(no. 2), a=6.466(1), b=8.822(1), c=17.548(1) Å, α=103.45(1), β=90.38(1), γ=100.97(1)°, V=954.4(2) Å3, Z=2, ρcalcd=2.165 g cm−3, T=150 K, μ(MoKα)=78.4 cm−1, F(000)=596. Reflections measured 19 839, independent 5795 with Rint=0.043. Final R2 (F 2, all reflections)=0.113, wR2=0.165, conventional R1=0.052 for 262 variables. Bruker SMART CCD area detector, MoKα radiation (λ=0.71073 Å), ω-scan mode, θmin=3°, θmax=26°. Structure solved by direct methods and refined by full-matrix least squares. The program used to refine the structure was Personal SDP. CCDC-272640 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18A. A. Zlota, F. Frolow, D. Milstein, J. Am. Chem. Soc. 1990, 112, 6411–6413; M. J. Calhorda, A. M. Galvão, C. Ünaleroglu, A. A. Zlota, F. Frolow, D. Milstein, Organometallics 1993, 12, 3316–3325; and references therein.
- 19M. A. Cinellu, G. Minghetti, M. V. Pinna, S. Stoccoro, A. Zucca, M. Manassero, J. Chem. Soc. Dalton Trans. 2000, 1261–1265.
- 20M. A. Cinellu, G. Minghetti, S. Stoccoro, A. Zucca, M. Manassero, M. Sansoni, J. Chem. Soc. Dalton Trans. 1996, 4217–4225.
- 214 a-PF6 was isolated in low yield after elaborate workup of the mixture. 4 a-BPh4 which conversely can be obtained in fairly good yields, is not stable enough in solution for carrying out a reactivity study.[15]
- 22General trend: 1) high 1 a-(PF6)2 conversions (high 3 a + 4 a yields) require a long reaction time (at least 15 days) a temperature of 10–15 °C and high nb/1 a ratio (20:1–30:1) both in MeCN and in MeCN/H2O. 2) Under the same preparative conditions the overall yield (3 a + 4 a) is only slightly higher in MeCN/H2O; nevertheless, the 3 a/4 a ratios are remarkably different: 6.5:1 (averaged) in MeCN and 1.5:1 in MeCN/H2O. 3) Under the same nb/1 a ratio and time conditions higher 4 a yields are obtained from concentrated solutions; the overall yield (3 a + 4 a) was slightly lower than that from dilute solutions.
- 23Signals of the epoxide could not be detected in the spectrum of the reaction mixture (CD3CN) as they overlapped with those of 1 c, 3 c, and 4 c.
- 241H NMR spectra were recorded soon after workup of the reaction mixture. Aldehyde signals (CDCl3): 7: 9.64 (d, 2.0 Hz; 2 H); 8 or 9: 9.63 (d, 2.4 Hz; 1 H); 9 or 8: 9.62 ppm (d, 2.4 Hz; 1 H). Intensities: 7≥(8 + 9); 7≤6 (molar ratio based on CHH-7 of the epoxide at δ=0.70 ppm).
- 251H NMR and GC-MS spectra were compared to those of an authentic sample prepared according to: S. Göksu, R. Altundas, Y. Sütbeyaz, Synth. Commun. 2000, 30, 1615–1621.
- 26Oxidation of nb with N2O to give 3-methylenecyclopentane carbaldehyde (and norbornanone) has recently been reported: E. V. Starokon, K. A. Dubkov, D. E. Babushkin, V. N. Parmon, G. I. Panov, Adv. Synth. Catal. 2004, 346, 268–274. In default of 1H NMR data for these aldehydes, the experimental spectra have been compared to the simulated ones obtained by ChemDraw 9.0 (CambridgeSoft).
- 27Complex 3 a and 6 also form upon addition of nb and a catalytic amount (5 %) of HBF4⋅Et2O to 4 a; in this case small amounts of diols 10 and 11 are formed.
- 28D. Milstein, J. C. Calabrese, J. Am. Chem. Soc. 1982, 104, 3773–3774.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.