Ruthenium-Catalyzed Oxidative Cyanation of Tertiary Amines with Hydrogen Peroxide and Sodium Cyanide†
Shun-Ichi Murahashi Prof. Dr.
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
Present address: Department of Applied Chemistry, Faculty of Engineering, Okayama University of Science, 1–1 Ridaicho, Okayama, Okayama 700-0005, Japan, Fax: (+81) 86-256-9513
Search for more papers by this authorNaruyoshi Komiya Dr.
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
Search for more papers by this authorHiroyuki Terai
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
Search for more papers by this authorShun-Ichi Murahashi Prof. Dr.
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
Present address: Department of Applied Chemistry, Faculty of Engineering, Okayama University of Science, 1–1 Ridaicho, Okayama, Okayama 700-0005, Japan, Fax: (+81) 86-256-9513
Search for more papers by this authorNaruyoshi Komiya Dr.
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
Search for more papers by this authorHiroyuki Terai
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Graphical Abstract
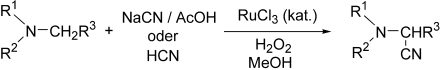
Vielseitige Zwischenstufen zur Synthese von N-Aryl-α-aminosäuren und N,N-disubstituierten 1,2-Diaminen sind durch Ruthenium-katalysierte oxidative Cyanierung von tertiären Aminen zugänglich. Die Verwendung von Wasserstoffperoxid als Oxidationsmittel in Gegenwart von NaCN/AcOH oder HCN liefert die entsprechenden α-Aminonitrile (siehe Schema).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z501496_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS.-I. Murahashi,N. Komiya, Ruthenium in Organic Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2004, pp. 53–93;
10.1002/3527603832.ch3 Google Scholar
- 1bS.-I. Murahashi,Y. Imada, Transition Metals for Organic Synthesis, Vol. 2 (Eds. ), Wiley-VCH, Weinheim, 1998, pp. 373–383;
- 1c Cytochrome P450, Structure, Mechanism and Biochemistry, 2nd ed. ), Plenum, New York, 1995;
- 1dC. Geoffrey, Introduction to Alkaloids, Wiley, New York, 1981.
- 2S.-I. Murahashi, Angew. Chem. 1995, 107, 2670;
10.1002/ange.19951072205 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2443.
- 3S.-I. Murahashi, T. Naota, K. Yonemura, J. Am. Chem. Soc. 1988, 110, 8256.
- 4
- 4aS.-I. Murahashi, T. Naota, T. Kuwabara, T. Saito, H. Kumobayashi, S. Akutagawa, J. Am. Chem. Soc. 1990, 112, 7820;
- 4bS.-I. Murahashi, T. Saito, T. Naota, H. Kumobayashi, S. Akutagawa, Tetrahedron Lett. 1991, 32, 5991.
- 5
- 5aS.-I. Murahashi, T. Hirano, T. Yano, J. Am. Chem. Soc. 1978, 100, 348;
- 5bS.-I. Murahashi, T. Watanabe, J. Am. Chem. Soc. 1979, 101, 7429.
- 6
- 6aG. Strukul, Catalytic Oxidations with Hydrogen Peroxide as Oxidant, Kluwer, Dordrecht 1992;
- 6bC. W. Jones, Application of Hydrogen Peroxide and Derivatives, Royal Society of Chemistry, Cambridge 1999;
10.1039/9781847550132 Google Scholar
- 6cI. W. C. E. Arends, R. A. Sheldon, M. Wallau, U. Schuchardt, Angew. Chem. 1997, 109, 1190;
10.1002/ange.19971091104 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1144;
- 6dC. C. Romano, F. E. Kühn, W. A. Herrmann, Chem. Rev. 1997, 97, 3197;
- 6eI. W. C. E. Arends, R. A. Sheldon, Top. Catal. 2002, 19, 133;
- 6fB. S. Lane, K. Burgess, Chem. Rev. 2003, 103, 2457;
- 6gR. Noyori, M. Aoki, K. Sato, Chem. Commun. 2003, 1977.
- 7Oxidation of alkenes:
- 7aK. Sato, M. Aoki, R. Noyori, Science 1998, 281, 1646;
- 7bK. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi, N. Mizuno, Science 2003, 300, 964;
- 7cY. Ishii, K. Yamawaki, T. Ura, H. Yamada, T. Yoshida, M. Ogawa, J. Org. Chem. 1988, 53, 3587;
- 7dR. Neumann, M. Gara, J. Am. Chem. Soc. 1995, 117, 5066;
- 7eD. E. De Vos, J. L. Meinershagen, T. Bein, Angew. Chem. 1996, 108, 2355;
10.1002/ange.19961081911 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2211;
- 7fP. Battioni, J. P. Renaud, J. F. Bartoli, M. Reina-Arfiles, M. Fort, D. Mansuy, J. Am. Chem. Soc. 1988, 110, 8462;
- 7gV. Venturello, E. Alneri, M. Ricci, J. Org. Chem. 1983, 48, 3831; oxidation of alcohols:
- 7hK. Sato, M. Aoki, J. Takagi, R. Noyori, J. Am. Chem. Soc. 1997, 119, 12386;
- 7iG. Barak, J. Dakka, Y. Sasson, J. Org. Chem. 1988, 53, 3553;
- 7jS. E. Jacobson, D. A. Muccigrosso, F. Mares, J. Org. Chem. 1979, 44, 921;
- 7kO. Bortoline, V. Cobnte, F. D. Furia, G. Modena, J. Org. Chem. 1986, 51, 2661;
- 7lR. Zennaro, F. Pinna, G. Strukul, H. Arzoumanian, J. Mol. Catal. 1991, 70, 269;
- 7mC. Venturello, M. Gambaro, J. Org. Chem. 1991, 56, 5924;
- 7nA. C. Dangel, W. P. Griffith, B. C. Parkin, J. Chem. Soc. Dalton Trans. 1993, 2683; oxidation of secondary amines:
- 7oH. Mitsui, S. Zenki, T. Shiota, S.-I. Murahashi, J. Chem. Soc. Chem. Commun. 1984, 874;
- 7pS.-I. Murahashi, H. Mitsui, T. Shiota, T. Tsuda, S. Watanabe, J. Org. Chem. 1990, 55, 1736;
- 7qS.-I. Murahashi, T. Shiota, Tetrahedron Lett. 1987, 28, 2383;
- 7rS.-I. Murahashi, T. Naota, N. Miyaguchi, T. Nakato, Tetrahedron Lett. 1992, 33, 6991; oxidation of alkanes:
- 7sD. H. R. Barton, D. Doller, Acc. Chem. Res. 1992, 25 504;
- 7tA. Sobkowiak,H.-C. Tung,D. T. Sawyer, Progress in Organic Chemistry, Vol. 40 (Ed. ), Wiley, New York, 1992, pp. 291–352;
- 7uA. S. Goldstein, R. H. Beer, R. S. Drago, J. Am. Chem. Soc. 1994, 116, 2424;
- 7vN. Mizuno, C. Nozaki, I. Kiyoto, M. Misono, J. Am. Chem. Soc. 1998, 120, 9267.
- 8For the copper-catalyzed alkynylation of tertiary amines with tBuOOH, see Z. Li, C.-J. Li, J. Am. Chem. Soc. 2004, 126, 11810.
- 9D. Enders, J. P. Shilvock, Chem. Soc. Rev. 2000, 29, 359.
- 10
- 10aS.-I. Murahashi, N. Komiya, H. Terai, T. Nakae, J. Am. Chem. Soc. 2003, 125, 15312;
- 10bM. North, Angew. Chem. 2004, 116, 4218;
10.1002/ange.200301750 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4126.
- 11
- 11aT. Shono, T. Toda, N. Oshino, J. Am. Chem. Soc. 1982, 104, 2639;
- 11bG. T. Miwa, W. A. Garland, B. J. Hodshon, A. Y. H. Lu, D. B. Northrop, J. Biol. Chem. 1980, 255, 6049;
- 11cG. T. Miwa, J. S. Walsh, G. L. Kedderis, P. F. Hollenberg, J. Biol. Chem. 1983, 258, 14445.
- 12
- 12aJ. A. Thompson, J. L. Holtzman, Drug Metab. Dispos. 1974, 2, 577;
- 12bD. C. Heimbrook, R. I. Murray, K. D. Egeberg, S. G. Sligar, M. W. Nee, T. C. Bruice, J. Am. Chem. Soc. 1984, 106, 1514.
- 13C. F. H. Allen, R. K. Kimball, Org. Synth. Coll. Vol. II 1943, p. 498.
- 14
- 14aN. Tanaka, T. Tamai, H. Mukaiyama, A. Hirabayashi, H. Muranaka, S. Akahane, H. Miyata, M. Akahane, J. Med. Chem. 2001, 44, 1436;
- 14bR. E. Webb, A. D. Johnston, J. Dent. Res. 1991, 70, 211.
- 15G. C. Barrett, Chemistry and Biochemistry of the Amino Acids (Ed.: ), Chapman and Hall, London, 1985, pp. 246–296.
10.1007/978-94-009-4832-7_8 Google Scholar
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.