Regio- and Chemoselective 6′-N-Derivatization of Aminoglycosides: Bisubstrate Inhibitors as Probes To Study Aminoglycoside 6′-N-Acetyltransferases†
This work was supported by the National Science and Engineering Research Council of Canada (NSERC), by the Canadian Institute of Health Research (CIHR), and by the Cancer Research Center (CRC). F.G., X.Y., and O.M.B. were supported by scholarship awards from the Chemical Biology Strategic Training Initiative of CIHR. The authors are grateful to G. D. Wright at McMaster University for sharing his AAC(6′)-Ii expression plasmid.
Graphical Abstract
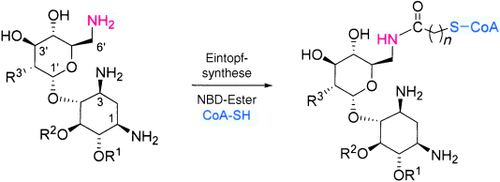
Einfach zu komplexen nanomolaren Inhibitoren: Aminoglycosid-Coenzym-A-Derivate wurden durch eine effiziente regioselektive 6′-N-Modifizierung von Aminoglycosiden hergestellt (siehe Schema). Diese Bisubstrate sind fest bindende kompetitive Inhibitoren der Aminoglycosid-6′-N-Acetyltransferase, eines an der Antibiotikaresistenz beteiligten Enzyms.