Direct Organocatalytic and Highly Enantio- and Diastereoselective Mannich Reactions of α-Substituted α-Cyanoacetates†
Thomas B. Poulsen
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorCarlos Alemparte Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorSteen Saaby Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorMarco Bella Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorKarl Anker Jørgensen Prof. Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorThomas B. Poulsen
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorCarlos Alemparte Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorSteen Saaby Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorMarco Bella Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorKarl Anker Jørgensen Prof. Dr.
The Danish National Research Foundation, Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax: (+45) 8919-6199
Search for more papers by this authorThis work was made possible by a grant from The Danish National Research Foundation. We are grateful to Dr. Jacob Overgaard for X-ray crystallographic analysis.
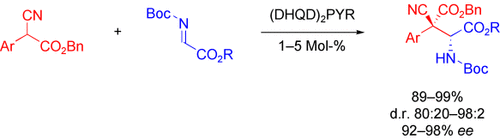
Graphical Abstract
Metallfreie Katalyse: Hoch funktionalisierte Moleküle mit zwei benachbarten Stereozentren sind mit einem käuflichen Organokatalysator einfach, in hoher Ausbeute und mit hoher Enantio- und Diastereoselektivität zugänglich (siehe Schema). Die leicht zu entfernende Boc-Schutzgruppe im Produkt ist ein zusätzlicher Vorteil dieser Methode.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z500144_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Arend, B. Westermann, N. Risch, Angew. Chem. 1998, 110, 1096–1122;
10.1002/(SICI)1521-3757(19980420)110:8<1096::AID-ANGE1096>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1044–1070;10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 1bE. F. Kleinmann in Comprehensive Organic Synthesis, Vol. 2 (Eds.: ), Pergamon, New York, 1991, Chap. 4.1.
10.1016/B978-0-08-052349-1.00052-4 Google Scholar
- 2For reviews on Mannich reactions with preformed enolates see:
- 2aS. Kobayashi, M. Ueno in Comprehensive Asymmetric Catalysis, Supplement, Vol. 1 (Eds.: ), Springer, Berlin, 2004, pp. 143–150;
- 2bS. Kobayashi, H. Ishitani, Chem. Rev. 1999, 99, 1069–1094;
- 2cS. E. Denmark, O. J.-C. Nicaise in Comprehensive Asymmetric Catalysis, Vol. 2 (Eds.: ), Springer, Berlin, 1999, pp. 923–961; for direct Mannich reactions, see:
- 2dA. Córdova, Acc. Chem. Res. 2004, 37, 102–112;
- 2eS. Matsunaga, T. Yoshida, H. Morimoto, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2004, 126, 8777–8785;
- 2fM. Marigo, A. Kjærsgaard, K. Juhl, N. Gathergood, K. A. Jørgensen, Chem. Eur. J. 2003, 9, 2359–2367;
- 2gB. M. Trost, L. Terrell, J. Am. Chem. Soc. 2003, 125, 338–339;
- 2hK. Juhl, N. Gathergood, K. A. Jørgensen, Angew. Chem. 2001, 113, 3083–3085;
10.1002/1521-3757(20010817)113:16<3083::AID-ANGE3083>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2995–2997.10.1002/1521-3773(20010817)40:16<2995::AID-ANIE2995>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 3For general reviews on organocatalysis, see:
- 3aP. I. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248–5286;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138–5175;
- 3bAcc. Chem. Res. 2004, 37(8) special issue (Eds.: K. N. Houk, B. List);
- 3cE. R. Jarvo, S. J. Miller, Tetrahedron 2002, 58, 2481–2495;
- 3dP. I. Dalko, L. Moisan, Angew. Chem. 2001, 113, 3840–3864;
10.1002/1521-3757(20011015)113:20<3840::AID-ANGE3840>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3726–3748.10.1002/1521-3773(20011015)40:20<3726::AID-ANIE3726>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 4
- 4aW. Notz, S.-I. Watanabe, N. S. Chowdari, G. Zhong, J. M. Betancort, F. Tanaka, C. F. Barbas III, Adv. Synth. Catal. 2004, 346, 1131–1140;
- 4bW. Zhuang, S. Saaby, K. A. Jørgensen, Angew. Chem. 2004, 116, 4576–4578; Angew. Chem. Int. Ed. 2004, 43, 4476–4478;
- 4cN. S. Chowdari, J. T. Suri, C. F. Barbas III, Org. Lett. 2004, 6, 2507–2510;
- 4dA. Córdova, Chem. Eur. J. 2004, 10, 1987–1997;
- 4eW. Notz, F. Tanaka, S. Watanabe, N. S. Chowdari, J. M. Turner, R. Thayumanavan, C. F. Barbas III, J. Org. Chem. 2003, 68, 9624–9634;
- 4fY. Hayashi, W. Tsuboi, I. Ashimine, T. Urushima, M. Shoji, K. Sakai, Angew. Chem. 2003, 115, 3805–3808; Angew. Chem. Int. Ed. 2003, 42, 3677–3680;
- 4gA. Córdova, W. Notz, G. Zhong, J. M. Betancort, C. F. Barbas III, J. Am. Chem. Soc. 2002, 124, 1866;
- 4hA. Córdova, F. Watanabe; S. Tanaka, W. Notz, C. F. Barbas III, J. Am. Chem. Soc. 2002, 124, 1842–1843;
- 4iB. List, P. Porjarliev, W. T. Biller, H. J. Martin, J. Am. Chem. Soc. 2002, 124, 827–833;
- 4jB. List, J. Am. Chem. Soc. 2000, 122, 9336–9337.
- 5General reviews:
- 5aS.-K. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621–631;
- 5bK. Kacprzak, J. Gawroński, Synthesis 2001, 961–998.
- 6S. France, D. J. Guerin, S. J. Miller, T. Lectka, Chem. Rev. 2003, 103, 2985–3012.
- 7
- 7aT. Ooi, K. Maruoka, Acc. Chem. Res. 2004, 37, 526–533;
- 7bB. Lygo, B. I. Andrews, Acc. Chem. Res. 2004, 37, 518–525;
- 7cM. J. O'Donnell, Acc. Chem. Res. 2004, 37, 506–517;
- 7dC. Carter, A. Nelson in Organic Synthesis Highlights V (Eds.: ), Wiley-VCH, Weinheim, 2003, pp. 125–133;
- 7eK. Maruoka, T. Ooi, Chem. Rev. 2003, 103, 3013–3028.
- 8For recent examples, see:
- 8aH. Li, Y. Wang, L. Tang, F. Wu, X. Liu, C. Guo, B. M. Foxman, L. Deng, Angew. Chem. 2005, 117, 107–110; Angew. Chem. Int. Ed. 2005, 44, 105–108;
- 8bX. Liu, H. Li, L. Deng, Org. Lett. 2005, 7, 167–169;
- 8cM. R. Acocella; O. García Mancheño, M. Bella, K. A. Jørgensen, J. Org. Chem. 2004, 69, 8165–8167;
- 8dS. Saaby, M. Bella, K. A. Jørgensen, J. Am. Chem. Soc. 2004, 126, 8120–8121;
- 8eM. Bella, K. A. Jørgensen, J. Am. Chem. Soc. 2004, 126, 5672–5673;
- 8fH. Li, Y. Wang, L. Tang, L. Deng, J. Am. Chem. Soc. 2004, 126, 9906–9907;
- 8gP. McDaid, Y. Cheng, L. Deng, Angew. Chem. 2002, 114, 348–350;
10.1002/1521-3757(20020118)114:2<348::AID-ANGE348>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 338–340;10.1002/1521-3773(20020118)41:2<338::AID-ANIE338>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 8hH. Brunner, P. Schmidt, Eur. J. Org. Chem. 2000, 2119–2133;
- 8iR. Alvarez, M.-A. Hourdin, C. Cavé, J. d′Angelo, P. Chaminade, Tetrahedron Lett. 1999, 40, 7091–7094.
- 9
- 9aJ. R. Dimmock, P. Kumar, Curr. Med. Chem. 1997, 4, 1–22;
- 9b Enantioselective Synthesis of β-Amino Acids (Ed.: ), Wiley-VCH, Weinheim, 1997.
- 10For examples, see:
- 10aR. B. Grossman, S. Comesse, R. M. Rasne, K. Hattori, M. N. Delong, J. Org. Chem. 2003, 68, 871–874, and references therein. For successful examples, see:
- 10bM. S. Taylor, E. N. Jacobsen, J. Am. Chem. Soc. 2003, 125, 11 204–11 205; refs. [8a,b,d]; see also:
- 10cN. Shibata, E. Suzuki, T. Asahi, M. Shiro, J. Am. Chem. Soc. 2001, 123, 7001–7009.
- 11For recent reviews on asymmetric construction of quaternary carbon atoms, see:
- 11aC. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 5363–5367;
- 11bJ. Christoffers, A. Baro, Angew. Chem. 2003, 115, 1726–1728;
10.1002/ange.200201614 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1688–1690;
- 11cE. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 402–415;
10.1002/(SICI)1521-3757(19980216)110:4<402::AID-ANGE402>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 388–401.10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google Scholar
- 12Changing the imine ester group (Me, Et, nPr, iPr, tBu) gave enantioselectivities for the preferred diastereomer above 90 % ee. The d.r. ranged between 80:20–89:11, the highest value having been observed for ethyl.
- 13
- 13aH. Hiemstra, H. Wynberg, J. Am. Chem. Soc. 1981, 103, 417–430;
- 13bK. Hermann, H. Wynberg, J. Org. Chem. 1979, 44, 2238–2244.
- 14The carbamate-protected imine was prepared in situ following the procedure developed by Kobayashi and co-workers; see:
- 14aY. Nakamura, R. Matsubara, H. Kiyohara, S. Kobayashi, Org. Lett. 2003, 5, 2481–2484;
- 14bS. Kobayashi, H. Kitagawa, R. Matsubara, J. Comb. Chem. 2001, 3, 401–403. The synthesis and use of α-bromo glycine derivatives in the preparation of acyl α-imino esters was initially developed by Steglich and co-workers; see:
- 14cR. Kober, W. Steglich, Liebigs Ann. Chem. 1983, 599–609;
- 14dP. Münster, W. Steglich, Synthesis 1987, 223–225.
- 15For a discussion of the difficulties in the deprotection of tosyl amines, see: R. R. Milburn, V. Snieckus, Angew. Chem. 2004, 116, 910–912;
10.1002/ange.200352634 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 892–894.
- 16As noted (Table 2) the reactions were performed on a 0.1-mmol scale. Compound 3 h was also prepared on a 0.5-mmol scale under the optimized conditions with similar results [95 % yield, 85:15 (u/l), and 95 % ee].
- 17The terms u (unlike) and l (like) were introduced by Seebach and Prelog as a general way of designating relative configurations in molecules with several chiral centers, particularly those in which terms like syn/anti or erythro/threo are not easily adopted; see: D. Seebach, V. Prelog, Angew. Chem. 1982, 94, 696; Angew. Chem. Int. Ed. Engl. 1982, 21, 654–660.
- 18Up to 61:39 (d.r.) and up to 51 % ee. This fact has also been observed in other cinchona alkaloid-catalyzed reactions of α-substituted cyanoacetates; see refs. [8b,d].
- 19CCDC 260994 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.