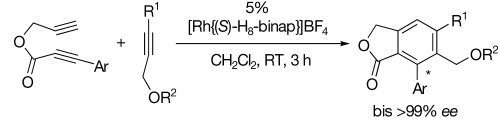
Enantioselective Synthesis of Axially Chiral Phthalides through Cationic [RhI(H8-binap)]-Catalyzed Cross Alkyne Cyclotrimerization†
Ken Tanaka Prof. Dr.
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan, Fax: (+81) 42-388-7037
Search for more papers by this authorGoushi Nishida
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan, Fax: (+81) 42-388-7037
Search for more papers by this authorAzusa Wada
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan, Fax: (+81) 42-388-7037
Search for more papers by this authorKeiichi Noguchi Prof. Dr.
Instrumentation Analysis Center, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588, Japan
Search for more papers by this authorKen Tanaka Prof. Dr.
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan, Fax: (+81) 42-388-7037
Search for more papers by this authorGoushi Nishida
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan, Fax: (+81) 42-388-7037
Search for more papers by this authorAzusa Wada
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan, Fax: (+81) 42-388-7037
Search for more papers by this authorKeiichi Noguchi Prof. Dr.
Instrumentation Analysis Center, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588, Japan
Search for more papers by this authorThis work was supported by Mitsubishi Chemical Corporation Fund and Asahi Glass Fund. We thank Takasago International Corporation for the gift of H8-binap.
Graphical Abstract
Einen einfachen Zugang zu axial-chiralen Phthaliden mit ein oder zwei Oxymethylen-Funktionalitäten bietet eine enantioselektive Alkincyclotrimerisierung in Gegenwart des kationischen Komplexes [RhI{(S)-H8-binap}]+. Die axiale Chiralität wird während der Bildung des Benzolrings mit hoher Enantioselektivität eingeführt.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461533_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Hayashi, K. Hayashizaki, Y. Ito, Tetrahedron Lett. 1989, 30, 215;
- 1bT. Hayashi, K. Hayashizaki, T. Kiyoi, Y. Ito, J. Am. Chem. Soc. 1988, 110, 8153.
- 2
- 2aJ. Yin, S. L. Buchwald, J. Am. Chem. Soc. 2000, 122, 12 051;
- 2bA. N. Cammidge, K. V. L. Crépy, Chem. Commun. 2000, 1723.
- 3
- 3aX. Li, J. B. Hewgley, C. A. Mulrooney, J. Yang, M. C. Kozlowski, J. Org. Chem. 2003, 68, 5500;
- 3bZ. Luo, Q. Liu, L. Gong, X. Cui, A. Mi, Y. Jiang, Chem. Commun. 2002, 914;
- 3cC. -Y, Chu, D.-R. Hwang, S.-K. Wang, B.-J. Uang, Chem. Commun. 2001, 980;
- 3dS. Saito, T. Kano, H. Muto, H. Nakadai, H. Yamamoto, J. Am. Chem. Soc. 1999, 121, 8943;
- 3eM. Nakajima, K. Kanayama, I. Miyoshi, S.-I. Hashimoto, Tetrahedron Lett. 1995, 36, 9519.
- 4
- 4aT. Kamikawa, T. Hayashi, Tetrahedron 1999, 55, 3455;
- 4bT. Hayashi, S. Niizuma, T. Kamikawa, N. Suzuki, Y. Uozumi, J. Am. Chem. Soc. 1995, 117, 9101.
- 5
- 5aY.-H. Cho, A. Kina, T. Shimada, T. Hayashi, J. Org. Chem. 2004, 69, 3811;
- 5bT. Shimada, Y.-H. Cho, T. Hayashi, J. Am. Chem. Soc. 2002, 124, 13 396.
- 6For the synthesis of axially chiral biaryl compounds through chirality exchange from sp3 central chirality to axial chirality, see: Y. Nishi, K. Wakasugi, K. Koga, Y. Tanabe, J. Am. Chem. Soc. 2004, 126, 5358.
- 7For the synthesis of axially chiral biaryl compounds by utilizing a planar chiral arene–chromium complex, see:
- 7aT. Watanabe, Y. Tanaka, R. Shoda, R. Sakamoto, K. Kamikawa, M. Uemura, J. Org. Chem. 2004, 69, 4152;
- 7bK. Kamikawa, T. Sakamoto, Y. Tanaka, M. Uemura, J. Org. Chem. 2003, 68, 9356, and references therein.
- 8For reviews, see:
- 8aS. Saito, Y. Yamamoto, Chem. Rev. 2000, 100, 2901;
- 8bM. Lautens, W. Klute, W. Tam, Chem. Rev. 1996, 96, 49.
- 9X. Zhang, K. Mashimo, K. Koyano, N. Sayo, H. Kumobayashi, S. Akutagawa, H. Takaya, Tetrahedron Lett. 1991, 32, 7283.
- 10K. Tanaka, K. Shirasaka, Org. Lett. 2003, 5, 4697.
- 11For the [RhCl(PPh3)3]-catalyzed non-asymmetric synthesis of phthalides with acetylene gas, see: B. Witulski, A. Zimmermann, Synlett 2002, 1855.
- 12For the nickel-catalyzed non-asymmetric synthesis of phthalides, see: Y. Sato, K. Ohashi, M. Mori, Tetrahedron Lett. 1999, 40, 5231.
- 13For the nickel-catalyzed asymmetric construction of sp3 central chirality, see: Y. Sato, T. Nishimata, M. Mori, Heterocycles 1997, 44, 443.
- 14For the nickel-catalyzed asymmetric synthesis of a chiral helicene, see: I. G. Stara, I. Stary, A. Kollarovic, F. Teply, S. Vyskocil, D. Saman, Tetrahedron Lett. 1999, 40, 1993.
- 15A. Gutnov, B. Heller, C. Fischer, H.- J. Drexler, A. Spannenberg, B. Sundermann, C. Sundermann, Angew. Chem. 2004, 116, 3883;
10.1002/ange.200454164 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3795.
- 16T. Shibata, T. Fujimoto, K. Yokota, K. Takagi, J. Am. Chem. Soc. 2004, 126, 8382.
- 17Shibata et al. reported one example of an asymmetric [2+2+2] cycloaddition of an unsymmetrical α,ω-diyne and a symmetrical monoyne (neutral [IrI(Et-duphos)] catalyst, 81 % ee).[16]
- 18CCDC-246 690 (3 dd) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.