Stereospecific, Enantioselective Allylation of α-Hydrazono Esters by Using Allyltrichlorosilanes with BINAP Dioxides as Neutral-Coordinate Organocatalysts†
Chikako Ogawa Dr.
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5684-0634
Search for more papers by this authorMasaharu Sugiura Dr.
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5684-0634
Search for more papers by this authorShū Kobayashi Prof. Dr.
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5684-0634
Search for more papers by this authorChikako Ogawa Dr.
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5684-0634
Search for more papers by this authorMasaharu Sugiura Dr.
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5684-0634
Search for more papers by this authorShū Kobayashi Prof. Dr.
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Fax: (+81) 3-5684-0634
Search for more papers by this authorThis work was partially supported by CREST, SORT, ERATO, the Japan Science Technology Corporation (JST), and a Grant-in-Aid for Scientific Research from the Japan Society of the Promotion of Sciences (JSPS). BINAP=2,2′-Bis(diphenylphosphanyl)-1,1′-binaphthyl.
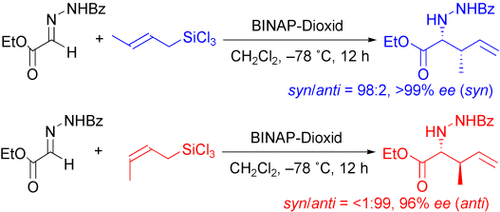
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461312_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. Ferraris, T. Dudding, B. Young, W. J. Drurry III, T. Lectka, J. Org. Chem. 1999, 64, 2168;
- 1bD. Ferraris, B. Young, C. Cox, T. Dudding, W. J. Drury III, L. Ryzhkov, A. E. Taggi, T. Lectka, J. Am. Chem. Soc. 2002, 124, 67.
- 2X. Fang, M. Johannsen, S. Yao, N. Gathergood, R. G. Hazel, K. A. Jørgensen, J. Org. Chem. 1999, 64, 4844.
- 3T. Hamada, K. Manabe, S. Kobayashi, Angew. Chem. 2003, 115, 4057;
10.1002/ange.200351778 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3927.
- 4For a diastereo- and enantioselective allylation of imines, see: T. Gastner, H. Ishitani, R. Akiyama, S. Kobayashi, Angew. Chem. 2001, 113, 1949;
10.1002/1521-3757(20010518)113:10<1949::AID-ANGE1949>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1896.10.1002/1521-3773(20010518)40:10<1896::AID-ANIE1896>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 5
- 5aS. Kobayashi, K. Nishio, Tetrahedron Lett. 1993, 34, 3453;
- 5bS. Kobayashi, K. Nishio, J. Org. Chem. 1994, 59, 6620.
- 6
- 6aS. Kobayashi, R. Hirabayashi, J. Am. Chem. Soc. 1999, 121, 6942;
- 6bR. Hirabayashi, C. Ogawa, M. Sugiura, S. Kobayashi, J. Am. Chem. Soc. 2001, 123, 9493;
- 6cC. Ogawa, M. Sugiura, S. Kobayashi, J. Org. Chem. 2002, 67, 5359.
- 7
- 7aC. C. Chuit, R. J. Corriu, C. Reye, J. C. Young, Chem. Rev. 1993, 93, 1371;
- 7bH. Sakurai, Synlett 1989, 1.
- 8S. Kobayashi, M. Sugiura, C. Ogawa, Adv. Synth. Catal. 2004, 346, 1023.
- 9S. Kobayashi, C. Ogawa, H. Konishi, M. Sugiura, J. Am. Chem. Soc. 2003, 125, 6610.
- 10C. Ogawa, H. Konishi, M. Sugiura, S. Kobayashi, Org. Biomol. Chem. 2004, 2, 446.
- 11
- 11aK. Manabe, H. Oyamada, K. Sugita, S. Kobayashi, J. Org. Chem. 1999, 64, 8054;
- 11bS. Kobayashi, T. Hamada, K. Manabe, J. Am. Chem. Soc. 2002, 124, 5640.
- 12
- 12aK. Mikami, M. Yamaoka, Tetrahedron Lett. 1998, 39, 4501;
- 12bQ.-H. Fan, G.-H. Liu, G.-J. Deng, X.-M. Chen, A. S. C. Chan, Tetrahedron Lett. 2001, 42, 9047;
- 12cJ. Bayaedon, M. Cavazzini, D. Maikkard, G. Pozzi, S. Quici, D. Sinou, Tetrahedron: Asymmetry 2003, 14, 2215;
- 12dA. M. Maj, K. M. Pietrusiewicz, I. Suisse, F. Agbossou, A. Mortreux, Tetrahedron: Asymmetry 1999, 10, 831;
- 12eS. Matsukawa, H. Sugama, T. Imamoto, Tetrahedron Lett. 2000, 41, 6461.
- 13 Catalytic Asymmetric Synthesis (Ed.: ), 2nd ed., Wiley-VCH, New York, 2000.
10.1002/0471721506 Google Scholar
- 14The stereochemistry of the adducts was tentatively assigned at this stage based on previous data (see ref [3]). It was finally confirmed after converting to D-alloisoleucine (Scheme 1).
- 15See, for example: K. Takesato, K. Ikai, F. Haruna, M. Endo, K. Shimanaka, E. Sono, T. Nakamura, I. Kato, J. Antibiot. 1991, 44, 919.
- 16
- 16aW. Oppolzer, O. Tamura, Tetrahedron Lett. 1990, 31, 991;
- 16bM. Bakke, H. Ohta, U. Kazmaier, T. Sugai, Synthesis 1999, 9, 1671;
10.1055/s-1999-3565 Google Scholar
- 16cP. Portonovo, B. Liang, M. Joullie, Tetrahedron: Asymmetry 1999, 10, 1451;
- 16dX. Wang, M. Xu, J. Chen, Y. Pan, Y. Shi, Synth. Commun. 2000, 30, 2253;
- 16eW. Oppolzer, R. Pedrosa, R. Moretti, Tetrahedron Lett. 1986, 27, 831;
- 16fW. Oppolzer, O. Tamura, J. Deeberg, Helv. Chim. Acta 1992, 75, 1965;
- 16gP. Lloyd-William, P. Monerris, I. Gonzalez, G. Jou, E. Giralt, J. Chem. Soc. Perkin Trans. 1 1994, 1969;
- 16hH. Noda, K. Sakai, H. Murakami, Tetrahedron: Asymmetry 2002, 13, 2649.
- 17R. Akiyama, S. Kobayashi, J. Am. Chem. Soc. 2003, 125, 3412.
- 18M. J. Burk, J. E. Feaster, J. Am. Chem. Soc. 1992, 114, 6266.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.