Catalytic Asymmetric Mercuriocyclization of γ-Hydroxy-cis-Alkenes†
Sung Ho Kang Prof. Dr.
Center for Molecular Design and Synthesis, Department of Chemistry, School of Molecular Science (BK21), Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea, Fax: (+82) 42-869-2810
Search for more papers by this authorMihyong Kim Dr.
Center for Molecular Design and Synthesis, Department of Chemistry, School of Molecular Science (BK21), Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea, Fax: (+82) 42-869-2810
Search for more papers by this authorSuk Youn Kang Dr.
Center for Molecular Design and Synthesis, Department of Chemistry, School of Molecular Science (BK21), Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea, Fax: (+82) 42-869-2810
Search for more papers by this authorSung Ho Kang Prof. Dr.
Center for Molecular Design and Synthesis, Department of Chemistry, School of Molecular Science (BK21), Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea, Fax: (+82) 42-869-2810
Search for more papers by this authorMihyong Kim Dr.
Center for Molecular Design and Synthesis, Department of Chemistry, School of Molecular Science (BK21), Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea, Fax: (+82) 42-869-2810
Search for more papers by this authorSuk Youn Kang Dr.
Center for Molecular Design and Synthesis, Department of Chemistry, School of Molecular Science (BK21), Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea, Fax: (+82) 42-869-2810
Search for more papers by this authorThis work was supported by CMDS and the Brain Korea 21 Project.
Graphical Abstract
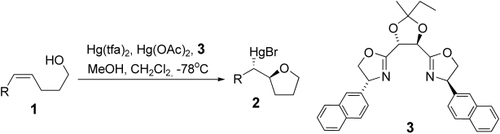
2-Monosubstituierte Tetrahydrofurane 2 entstehen mit 73–95 % ee bei der katalytischen enantioselektiven Mercuriocyclisierung von γ-Hydroxy-(Z)-alkenen 1 mit Hg(OAc)2 in Gegenwart von HgII, das mit dem Bisoxazolin 3 komplexiert ist. Der chirale Komplex wurde aus Hg(tfa)2 (0.2 Äquiv.) und 3 (0.3 Äquiv.) hergestellt. Die MeOH-Menge hat einen großen Einfluss auf die Enantioselektivität.
References
- 1
- 1aT. Katsuki in Comprehensive Asymmetric Catalysis; Vol. 2 (Eds.: ), Springer, Heidelberg, 1999, chap. 18.1;
- 1bE. N. Jacobsen, M. F. Wu in Comprehensive Asymmetric Catalysis, Vol. 2 (Eds.: ), Springer, Heidelberg, 1999, chap. 18.2;
- 1cR. W. Murray, Chem. Rev. 1989, 89, 1187.
- 2
- 2aI. E. Marko, J. S. Svendsen in Comprehensive Asymmetric Catalysis, Vol. 2 (Eds.: ), Springer, Heidelberg, 1999, chap. 20;
- 2bH. C. Kolb, M. S. Van Nieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483.
- 3
- 3aG. Li, H.-T. Chang, K. B. Sharpless, Angew. Chem. 1996, 108, 449; Angew. Chem. Int. Ed. Engl. 1996, 35, 451;
- 3bG. Li, H. H. Angert, K. B. Sharpless, Angew. Chem. 1996, 108, 2991; Angew. Chem. Int. Ed. Engl. 1996, 35, 2813.
- 4
- 4aR. Noyori, Angew. Chem. 2002, 114, 2108;
10.1002/1521-3757(20020617)114:12<2108::AID-ANGE2108>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2008;10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 4bW. S. Knowles Angew. Chem. 2002, 114, 2096; Angew. Chem. Int. Ed. 2002, 41, 1998.
- 5
- 5aF. Y. Kwong, Q. Yong, T. C. W. Mak, A. S. C. Chan, K. S. Chan, J. Org. Chem. 2002, 67, 2769, and references therein;
- 5bK. Burgess, W. A. Van der Donk in Advanced Asymmetric Synthesis (Ed.: ), Champman & Hall, London, 1996, p. 181.
10.1007/978-94-007-0797-9_9 Google Scholar
- 6
- 6aG. Rousseau, S. Robin, Tetrahedron 1998, 54, 13 681;
- 6bK. E. Harding, T. H. Tinger in Comprehensive Organic Synthesis, Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, p. 463;
- 6cJ. Mulzer in Organic Synthesis Highlights (Eds.: ), Wiley-VCH, Weinheim, 1991, p. 157.
- 7
- 7aT. G. Back, B. P. Dyck, S. Nan, Tetrahedron 1999, 55, 3191;
- 7bY. Nishibayashi, S. K. Srivastava, H. Takada, S.-I. Fukuzawa, S. Uemura, J. Chem. Soc. Chem. Commun. 1995, 2321;
- 7cT. Wirth, Angew. Chem. 1995, 107, 1872; Angew. Chem. Int. Ed. Engl. 1995, 34, 1726;
- 7dR. Déziel, S. Goulet, L. Grenier, J. Bordeleau, J. Nernier, J. Org. Chem. 1993, 58, 3619;
- 7eK.-I. Fujita, M. Iwaoka, S. Tomoda, Chem. Lett. 1992, 1123.
- 8
- 8aR. B. Grossman, R. J. Trupp, Can. J. Chem. 1998, 76, 1233;
- 8bH. B. Vardhan, R. D. Bach, J. Org. Chem. 1992, 57, 4948.
- 9S. H. Kang, S. B. Lee, C. M. Park, J. Am. Chem. Soc. 2003, 125, 15 748.
- 10S. H. Kang, M. Kim, J. Am. Chem. Soc. 2003, 125, 4684.
- 11When the cyclization of 1 was scaled up from 0.2 to 2.0 mmol, the enantioselectivity decreased to 90 % ee. However, the addition of 1 by a syringe pump over 8 h instead of in one portion restored it to the initial level (93 % ee).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.