Organic-Based Layered Perovskites of Mixed-Valent Gold(I)/Gold(III) Iodides†
Laura M. Castro-Castro
Department of Chemistry, University of Houston, Houston, Texas, 77204–5641, USA, Fax: (+1) 713-743–2787
Search for more papers by this authorArnold M. Guloy Prof.
Department of Chemistry, University of Houston, Houston, Texas, 77204–5641, USA, Fax: (+1) 713-743–2787
Search for more papers by this authorLaura M. Castro-Castro
Department of Chemistry, University of Houston, Houston, Texas, 77204–5641, USA, Fax: (+1) 713-743–2787
Search for more papers by this authorArnold M. Guloy Prof.
Department of Chemistry, University of Houston, Houston, Texas, 77204–5641, USA, Fax: (+1) 713-743–2787
Search for more papers by this authorThis work was supported by the R.A. Welch Foundation, the Texas Center for Superconductivity at UH, and the National Science Foundation (CAREER Award, DMR-9733587). The authors are also grateful to Prof. P. S. Halasyamani for the TGA measurements.
Graphical Abstract
Blattgold: In neuartigen gemischtvalenten AuI/AuIII-Perowskitstrukturen werden Schichten aus [AuI2]−- und [AuI4]−-Einheiten durch I3−-Ionen und geeignete organische Dikationen zu dreidimensionalen Architekturen verbunden. Die experimentell bestimmten optischen Bandlücken dieser organisch-anorganischen Hybridhalbleiter sind kleiner als in Cs2Au2I6.
References
- 1
- 1aP. Day, Philos. Trans. R. Soc. London Ser. A 1985, 314, 145;
- 1bJ. Takada, H. Awaji, M. Koshioka, A. Nakajima, W. A. Nevin, Appl. Phys. Lett. 1992, 61, 2184;
- 1cP. G. Lacroix, R. Clement, K. Nakatani, J. Zyss, I. Ledoux, Science 1994, 263, 658;
- 1dL. Ouahab, Chem. Mater. 1997, 9, 1909.
- 2
- 2aD. B. Mitzi, S. Wang, C. A. Feild, C. A. Chess, A. M. Guloy, Science 1995, 267, 1473;
- 2bD. B. Mitzi, Prog. Inorg. Chem. 1999, 48, 1;
- 2cC. R. Kagan, D. B. Mitzi, C. D. Dimitrakopoulos, Science 1999, 286, 945.
- 3
- 3aJ.-M. Lehn, Supramolecular Chemistry, VCH, Weinheim, 1995;
10.1002/3527607439 Google Scholar
- 3bP. Metrangolo, G. Resnati, Chem. Eur. J. 2001, 7, 2511;
10.1002/1521-3765(20010618)7:12<2511::AID-CHEM25110>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 3cD. Quiñonero, C. Garau, C. Rotger, A. Frontera, P. Ballester, A. Costa, P. M. Deyà, Angew. Chem. 2002, 114, 3539–3542;
10.1002/1521-3757(20020916)114:18<3539::AID-ANGE3539>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3389–3393.10.1002/1521-3773(20020916)41:18<3389::AID-ANIE3389>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 4
- 4aA. J. Blake, R. O. Gould, S. Parsons, C. Radek, M. Schröder, Angew. Chem. 1995, 107, 2563;
10.1002/ange.19951072108 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2374;
- 4bZ. Tang, A. M. Guloy, J. Am. Chem. Soc. 1999, 121, 452;
- 4cA. M. Guloy, Z. Tang, P. B. Miranda, V. I. Srdanov, Adv. Mater. 2001, 13, 833;
- 4dT. Hertzsch, F. Budde, E. Weber, J. Hulliger, Angew. Chem. 2002, 114, 2385–2388;
10.1002/1521-3757(20020703)114:13<2385::AID-ANGE2385>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2281–2284.
- 5
- 5aG. A. Landrum, R. Hoffmann, Angew. Chem. 1998, 110, 1989–1992;
10.1002/(SICI)1521-3757(19980703)110:13/14<1989::AID-ANGE1989>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1887–1890;10.1002/(SICI)1521-3773(19980803)37:13/14<1887::AID-ANIE1887>3.0.CO;2-0 CAS Web of Science® Google Scholar
- 5bG. A. Landrum, N. Goldberg, R. Hoffmann, New J. Chem. 1998, 22, 883;
- 5cL. Kloo, J. Rosdahl, P. H. Svensson, Eur. J. Inorg. Chem. 2002, 1203.
- 6
- 6aR. J. Cava, B. Batlogg, J. J. Krajewski, R. Farrow, L. W. Rupp, A. E. White, K. Short, W. F. Peck, T. Kometani, Nature 1988, 332, 814;
- 6bA. W. Sleight, J. L. Gillson, P. E. Bierstedt, Solid State Commun. 1975, 17, 27.
- 7M. B. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1967, 10, 247.
- 8
- 8aN. Kojima, M. Hasegawa, H. Kitagawa, T. Kikegawa, O. Shimomura, J. Am. Chem. Soc. 1994, 116, 11 368;
- 8bN. Kojima, N. Matsushita, Coord. Chem. Rev. 2000, 198, 251.
- 9aH. L. Wells, Am. J. Sci. 1922, 3, 315;
- 9bH. L. Wells, Am. J. Sci. 1922, 3, 417;
- 9cN. Elliot, L. Pauling, J. Am. Chem. Soc. 1938, 60, 1846.
10.1021/ja01275a037 Google Scholar
- 10N. Matsushita, H. Kitagawa, N. Kojima, Acta Crystallogr. Sect. C 1997, 53, 663.
- 11D. Weber, Z. Naturforsch. B 1978, 33, 862.
- 12S. D. Gupta, J. Am. Chem. Soc. 1914, 36, 747.
- 13Crystallographic data for [NH3(CH2)8NH3]2[(AuI2)(AuI4)(I3)2]: monoclinic, space group C2/m (no.12), a=32.1710(6), b=7.7846(2), c=8.8640(2) Å, β=103.17(0)°, V=2161.46(2) Å3, ρcalc=3.395 g cm−3, Z=2, F(>4σ)=1908, λ=0.71073 Å, T=223 K, 6797 measured reflections, 2662 independent reflections. Single-crystal X-ray analysis was performed by using a Siemens SMART diffractometer equipped with a 1 K CCD area detector. Empirical absorption correction was applied by using ψ-scans. The structures were solved by direct methods and refined by full-matrix least-squares calculations. Thermal parameters of all non-H atoms were treated anisotropically. Hydrogen atoms were located at ideal positions using a riding model. All calculations were made by using the Siemens SHELXTL crystallographic package resulting in R(int)=0.0555, R1=0.0388, wR2=0.1006.[24]
- 14Crystallographic data for [NH3(CH2)7NH3]2[(AuI2)(AuI4)(I3)2]: triclinic, space group P
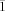 (no. 2), a=7.8197(1), b=8.6794(1), c=15.9093(2) Å, α=80.87(0)°, β=77.22(0)°, γ=89.50(0)°, V=1039.29(64) Å3, ρcalc=3.485 g cm−3, Z=1, F(>4σ)=2844, λ=0.71073 Å, T=223 K, 6478 measured reflections, 4490 symmetry-independent reflections. Empirical absorption correction was applied using SADABS. R(int)=0.0385, R1=0.0534, wR2=0.1423. CCDC-197203 and CCDC 197204 (1 and 2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
(no. 2), a=7.8197(1), b=8.6794(1), c=15.9093(2) Å, α=80.87(0)°, β=77.22(0)°, γ=89.50(0)°, V=1039.29(64) Å3, ρcalc=3.485 g cm−3, Z=1, F(>4σ)=2844, λ=0.71073 Å, T=223 K, 6478 measured reflections, 4490 symmetry-independent reflections. Empirical absorption correction was applied using SADABS. R(int)=0.0385, R1=0.0534, wR2=0.1423. CCDC-197203 and CCDC 197204 (1 and 2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 15J. Guan, Z. Tang, A. M. Guloy, Chem. Commun. 1999, 1833.
- 16
- 16aP. H. Svensson, J. Rosdahl, L. Kloo, Chem. Eur. J. 1999, 5, 305;
- 16bP. H. Svensson, G. Raud, L. Kloo, Eur. J. Inorg. Chem. 2000, 1275.
10.1002/(SICI)1099-0682(200006)2000:6<1275::AID-EJIC1275>3.3.CO;2-U CAS Web of Science® Google Scholar
- 17
- 17aN. Kojima, H. Kitagawa, J. Chem. Soc. Dalton Trans. 1994, 327;
- 17bH. Kitagawa, N. Kojima, T. Nakajima, J. Chem. Soc. Dalton Trans. 1991, 3121.
- 18Optical diffuse reflectance measurements were made at room temperature for pure samples of compounds 1 and 2 by using a Cary 500 double beam, double monochromator spectrometer. Several single crystals were placed on a sample holder within a Praying-Mantis apparatus. Polytetrafluoroethylene was used as a standard background reference (100 % reflectance). Absorption data were calculated from the reflectance data using the Kubelka-Munk function:[19] [α/S=(1−R)2/2 R], where R is the reflectance at a given wavelength, α is the absorption coefficient and S is the scattering coefficient. The electronic excitation energy, Eg, is extracted from the (α/S)2 versus energy plot.
- 19aW. W. Wendlandt, H. G. Hecht, Reflectance Spectroscopy, Interscience Publishers, New York, 1966;
- 19bG. Kotiim, Reflectance Spectroscopy, Springer, New York, 1969.
- 20X. L. Liu, K. Matsuda, Y. Moritomo, A. Nakamura, N. Kojima, Phys. Rev. B 1999, 59, 7925.
- 21Chemical analysis was performed by SEM. Crystalline samples were placed on an adhesive carbon tape attached to a brass disk. No carbon coating was necessary for these narrow band gap semiconductors. The samples were then bombarded with a 15 eV electron beam over at least 15 1-micron diameter areas, to determine the elements present and to then average the ratio of gold and iodine. Compositional precision was found to be within 5 % error. Data was collected by using a JEOL JSM 6400 scanning electron microscope equipped with a wave-dispersive spectrometer (WDS).
- 22The diamagnetic behavior of compound 1 (25 mg pellet sample) was examined by using an Oxford VSM instrument at a magnetic field of 0.01 T.
- 23
- 23aGaussian 98 (Revision A.7), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998;
- 23bC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785;
- 23cB. Miehlich, A. Savin, H. Stoll, H. Preuss, Chem. Phys. Lett. 1989, 157, 200;
- 23dA. D. Becke, J. Chem. Phys. 1993, 98, 5648;
- 23eT. Leininger, A. Nicklass, H. Stoll, M. Dolg, P. Schwerdtfeger, J. Chem. Phys. 1996, 105, 1052.
- 24G. M. Sheldrick, O. R. Gould, Acta Crystallogr. Sect. B 1995, 51, 423.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.





