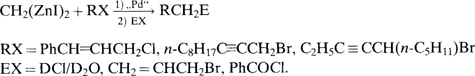Sukzessive Reaktion von Bis(iodzinkio)methan mit zwei unterschiedlichen Elektrophilen†
Diese Arbeit wurde vom Ministerium für Erziehung, Wissenschaft, Sport und Kultur unterstützt (Grant in Aid Nr. 06403025, 09231223, 09238221).
Abstract
[Pd2(dba)3] katalysiert in Gegenwart von Trifurylphosphan (TFP) die sukzessive Umsetzung von CH2(ZnI)2 mit verschiedenen Elektrophilen [G1. (a)]. Die homologe Zn2-Verbindung CH3CH(ZnI)2 reagiert im 1. Schritt analog mit Cinnamylchlorid; für die Kupplung mit Allylbromid im zweiten Schritt ist aber CuCN/LiCN in stöchiometrischen Mengen nötig. CH2(ZnI)2+RXRCH2ERXPhCHCHCH2Cl, n-C8H17CCCH2Br, C2H5CCCH(n-C5H11)Br EXDCl/D2O, CH2CHCH2Br, PhCOCl. (a)