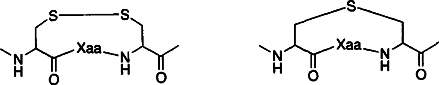β,β-Dimethylcyclolanthionine, neue Dipeptidmimetica mit geringer konformativer Freiheit: Synthese, Röntgenstruktur- und Konformationsanalyse†‡
Prof. Hui Shao
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorProf. Chang-Woo Lee
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorProf. Qin Zhu
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorDr. Peter Gantzel
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorCorresponding Author
Prof. Murray Goodman
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202Search for more papers by this authorProf. Hui Shao
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorProf. Chang-Woo Lee
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorProf. Qin Zhu
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorDr. Peter Gantzel
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Search for more papers by this authorCorresponding Author
Prof. Murray Goodman
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202
Department of Chemistry and Biochemistry University of California, San Diego La Jolla, CA 92093–0343 (USA) Telefax: Int. + 619/534–0202Search for more papers by this authorWir danken Dr. George Ösapay (UC, Riverside), Dr. Yunfei Zhu (Bioresearch Inc.) und Dr. Joseph Taulane für hilfreiche Diskussionen sowie Dr. Richard Kondrat (UC, Riverside) für die massenspektrometrischen Analysen. Diese Arbeit wurde gefördert durch NIHDK-15410.
Professor Ivar Ugi zum 65. Geburtstag gewidmet
Abstract
Zur Substitution der labilen Disulfidbrücken zwischen Cysteinresten in natürlichen Peptiden eignet sich Lanthionin (siehe unten), wie die Synthese konformativ rigider Dipeptidmimetica auf der Grundlage von β, β-Dimethylcyclolanthioninen belegt. Ihre strukturellen Eigenschaften zeigen, daß sie eine neue Familie von Peptidmimetica repräsentieren.
References
- 1 Für neuere Beispiele siehe a) M. Goodman, S. Ro, Burger's Medicinal Chemistry and Drug Discovery, Vol. 1 (Hrsg.: M. E. Wollf), Wiley, New York, 1995, S. 803–861; b) H. Shao, Q. Zhu, M. Goodman, J. Org. Chem. 1995, 60, 790–791; c) R. Hirschmann, K. C. Nicolaou, S. Pietranico, J. Salvino, E. M. Leahy, P. A. Sprengeler, G. Furst, A. B. Smith, C. D. Strader, M. A. Cascieri, M. R. Candelore, C. Donaldson, W. Vale, L. Maechler, J. Am. Chem. Soc. 1992, 114, 9217–9218; d) A. B. Smith, M. C. Guzman, P. A. Sprengeler, T. P. Keenan, R. C. Holcomb, J. L. Wood, P. J. Carroll, R. Hirschmann, J. Am. Chem. Soc. 1994, 116, 9947–9962; e) F. Cornille, U. Slomczynska, M. L. Smythe, D. D. Beusen, K. D. Moeller, G. R. Marshall, J. Am. Chem. Soc. 1995, 117, 909–917; f) A. Giannis, T. Kolter, Angew. Chem. 1993, 105, 1303–1326; Angew. Chem. Int. Ed. Engl. 1993, 32, 1244–1267; g) J. Gante, Angew. Chem. Int. Ed. Engl. 1994, 106, 1780 bzw. Angew. Chem. Int. Ed. Engl. 1994, 33, 1699–1720; h) V. J. Hruby, A. Gehring, Med. Res. Rev. 1989, 9, 343–401.
- 2a) Nisin and Novel Lantibiotics (Hrsg.: G. Jung, H. G. Sahl), Escom, Leiden, 1988; b) R. Zhang, G. H. Snyder, J. Biol. Chem. 1989, 264, 18472–18479, zit. Lit.; c) S. Chatterjee, D. K. Chatterjee, S. J. Lad, M. S. Phansalkar, R. H. Rupp, B. N. Ganguli, J. Antibiot. 1992, 45, 832–838; d) S. Chatterjee, D. K. Chatterjee, R. H. Jani, J. Blumbach, B. N. Ganguli, J. Antibiot 1992, 45, 839–845.
- 3a) H. Shao, S. H.-H. Wang, C.-W. Lee, G. Ösapay, M. Goodman, J. Org. Chem. 1995, 60, 2956–2957; b) G. Ösapay, M. Goodman, J. Chem. Soc. Chem. Commun. 1993, 1599–1600; c) A. Polinsky, M. G. Cooney, A. Toy-Palmer, G. Ösapay, M. Goodman, J. Med. Chem. 1992, 35, 4185–4194; d) G. Ösapay, H. Shao, M. Goodman, J. W. Taylor, Pept. Chem. Biol. Proc. Am. Pept. Symp. 13th 1994, 101–103; e) C.-W. Lee, Q. Zhu, H. Shao, S. H.-H. Wang, G. Ösapay, M. Goodman, Pept. Proc. Eur. Pept. Symp. 23rd 1994, 627–628; f) S. H.-H. Wang, Pept. Chem. Biol. Proc. Am. Pept. Symp. 14th 1995, im Druck; g) G. Ösapay, Q. Zhu, H. Shao, R. H. Chadha, M. Goodman, Int. J. Pept. Protein Res. 1995, 46, 290–301.
- 4a) E. E. Sugg, D. Tourwe, W. Kazmierski, V. J. Hruby, G. Van Binst, Int. J. Pept. Protein Res. 1988, 31, 192–200; b) S. Horvat, B. Gragas, N. Raog, V. Simeon, Int. J. Pept. Protein Res. 1989, 34, 346–351.
- 5a) D. K. Sukumaran, M. Prorok, D. S. Lawrence, J. Am. Chem. Soc. 1991, 113, 706–707; b) M. Bodanszky, G. L. Stahl, Proc. Natl. Acad. Sci. USA 1974, 71, 2791–2794; c) Y. A. Ovchinnikov, V. M. Lipkin, T. M. Shuvaeva, A. P. Bogachuk, V. V. Shemyakin, FEBS Lett. 1985, 179, 107; d) P. N. Kao, A. Karlin, J. Biol. Chem. 1986, 261, 8085–8088; e) S. M. Miller, M. J. Moore, V. Massey, C. Williams, M. D. Distefano, D. P. Ballou, C. T. Walsh, Biochemistry 1989, 28, 1194–1205.
- 6 R. Chandrasekaran, R. Balasubramanian, Biochim. Biophys. Acta 1969, 188, 1–9.
- 7a) S. Capasso, C. Mattia, L. Mazzarella, Acta Crystallogr. Sect. B 1977, 33, 2080–2083; b) Y. Hata, Y. Matsuura, N. Tanaka, T. Ashida, M. Kakudo, Acta Crystallogr. Sect. B 1977, 33, 3561–3564; c) A. Horne, M. North, J. A. Parkinson, I. H. Sadler, Tetrahedron 1993, 49, 5891–5904.
- 8 R. L. Baxter, S. S. B. Glover, E. M. Gordon, R. O. Gould, M. C. McKie, A. I. Scott, M. D. Walkinshaw, J. Chem. Soc. Perkin Trans. 1 1988, 365–371.
- 9 1: C17H22N2O5S, Kristalle aus Ethylacetat; monoklin, Raumgruppe P21, a = 12.041(6), b = 7.467(4), c = 22.20(1) Å, β = 118.59(4)°, Z = 2; Qber. = 1.273 gcm−3; äMo = 0.197 mm−1; 2162 gemessene Reflexe (I ≥ 4.0 σ(I)); 2 ϱmax = 55.0°; R = 0.0428; Rw = 0.0608; maximale Restelektronendichte 0.37 eÅ−3; CH in berechneten Positionen. 2: C17H22N2O5S, Kristalle aus Ethylacetat; monoklin, Raumgruppe P21, a = 6.228(3), b = 10.067(5), c = 14.548(6) Å, β = 90.89(3)°, Z = 2; Qber = 1.334 gcm−3; äMo = 0.207 mm−1; 2112 gemessene Reflexe (I ≥ 4.0 σ(I)); 2Θmax = 55.0°; R = 0.0505; Rw = 0.0704; maximale Restelektronendichte 0.34 eÅ−3; CH in berechneten Positionen. Verwendetes Programm: Siemens SHELXTL PLUS (PC Version). Weitere Einzelheiten zu den Kristallstrukturuntersuchungen können beim Direktor des Cambridge Crystallographic Data Centre, 12 Union Road, GB-Cambridge CB2 1EZ, unter Angabe des vollständigen Literaturzitats angefordert werden.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.