Fetal Intraparenchymal Hemorrhage Imaging Patterns, Etiology, and Outcomes: A Single Center Cohort Study
Abstract
Objective
This study examines associations among fetal brain magnetic resonance imaging (MRI) injury patterns, etiologies, and outcomes in fetal intraparenchymal hemorrhage (IPH).
Methods
This is a retrospective, single-center cohort study of IPH diagnosed on fetal MRI (1996–2022). IPH and associated abnormalities were categorized by 2 pediatric neuroradiologists; electronic medical records were reviewed by 2 pediatric neurologists to classify etiology and outcomes including cerebral palsy, epilepsy, developmental delay, and death.
Results
Forty-four fetuses with IPH were identified (34 singleton and 10 twin gestations) with MRI at median 24 weeks gestation (interquartile range [IQR] = 22–28 weeks). IPH was commonly supratentorial (84%) and focal (50%) or focal with diffuse injury (43%) and was often associated with germinal matrix hemorrhage (GMH; 75%) and/or intraventricular hemorrhage (IVH; 52%). An etiology was identified in 75%, including twin-twin transfusion syndrome (TTTS, n = 10), COL4A1/2 variants (n = 8), or other fetal/maternal conditions (n = 15). COL4A1/2 variants were associated with focal IPH and the presence of hemorrhagic porencephaly, and intrauterine transfusion was associated with infratentorial hemorrhage. Twenty-two fetuses were liveborn, and 18 pregnancies were terminated. Among those with follow-up ≥ 12 months (median = 7 years), 12 of 13 had cerebral palsy, 6 of 13 had developmental delay, and 5 of 13 had epilepsy.
Interpretation
An etiology for fetal IPH with or without GMH-IVH is identified in most cases in our cohort and is commonly TTTS, COL4A1/2 variants, or other maternal/fetal comorbidities. Pattern of fetal IPH on MRI is associated with etiology. Cerebral palsy and neurodevelopmental impairment were common in liveborn infants. Genetic studies should be considered in cases of fetal IPH without an otherwise apparent cause. ANN NEUROL 2024;96:1137–1147
Antenatal diagnosis of stroke has become more feasible with improved access to fetal magnetic resonance imaging (MRI); however, the epidemiology of fetal stroke and associated neurodevelopmental outcomes remain poorly understood. Most data on fetal stroke are grouped with perinatal stroke, defined as any ischemic or hemorrhagic stroke that occurs between 20 weeks gestational age (GA) in utero and 28 postnatal days.1-3 Perinatal stroke occurs in approximately 1 in 2,700 newborns,1 but the proportion of these cases that occur antenatally is not well known. Risk factors for perinatal hemorrhagic stroke include coagulation defects, fetal-neonatal alloimmune thrombocytopenia (FNAIT), COL4A1/2 variants, trauma, infection, and twin-twin transfusion syndrome (TTTS).4 Children with perinatal stroke have high rates of cerebral palsy (CP), cognitive or speech impairment, and epilepsy.4, 5 Previous cohorts of patients with perinatal hemorrhagic stroke presumably include a subset that occurred in utero, although this subset may have distinct characteristics and etiologies compared with infants with peripartum or neonatal hemorrhagic stroke.
The standard definition and presentation of stroke (acute-onset neurologic symptom attributable to brain infarct or hemorrhage) is not applicable to fetuses due to the inability to detect clinical symptoms. Hence, fetal stroke diagnosis requires imaging-based detection of ischemia or hemorrhage. Small areas of infarct or hemorrhage can be challenging to detect on fetal ultrasound (US) compared to MRI.6 Anatomy screening with US usually occurs between 18 and 22 weeks, and may miss the majority of perinatal strokes that typically occur in the late second or third trimesters.7 With improved prenatal imaging, particularly with fetal MRI, we can now better study fetal stroke, its patterns, epidemiology, and neurodevelopmental outcomes.
Prior studies on fetal hemorrhagic stroke rely primarily on fetal US, with limited use of fetal MRI.4, 8-11 Additionally, prior studies included fetuses with all types of intracranial hemorrhage (ICH), including isolated germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH), extra-axial hemorrhage, as well as intraparenchymal hemorrhage (IPH). Because the presence and extent of parenchymal hemorrhage is known to be a predictor of neurological outcome in preterm and term neonates,4, 9, 12 aggregating fetuses with IPH and those with isolated GMH-IVH limits the clinical utility of these data. Moreover, studies on etiology and outcome of fetal ICH tend to be enriched with isolated low-grade GMH-IVH, which is thought to rarely have an identifiable etiology and is typically associated with favorable outcomes.9 Consequently, there are a paucity of available data to guide prenatal diagnostic and prognostic counseling of expectant parents when a fetal IPH is identified.
There is a need for fetal MRI-based assessment of IPH to understand the patterns of injury and the association of imaging findings with both etiologies and clinical outcomes. This study leverages our large, longitudinal fetal MRI cohort to systematically evaluate the imaging findings, etiology, pregnancy outcome, and neurodevelopmental outcomes in fetuses with IPH with and without associated GMH-IVH. We hypothesize that imaging findings are associated with etiology of hemorrhage and with neurodevelopmental outcomes. Better understanding of fetal IPH is important for accurate diagnosis, improved prenatal counseling, and optimized clinical management.
Methods
We searched all fetal MRI reports at our institution from November 1996 to May 2022 for keywords “hemorrhage,” “hemosiderin,” “hematoma,” “hemorrhagic,” “stroke,” “infarct,” “schizencephaly,” “porencephaly,” “injury,” “ischemia,” “ischemic,” “blood products,” or “blood.” All identified reports were reviewed by a neuroradiologist to identify cases with definite or suspicion for fetal IPH based on report description. Cases with isolated GMH, extra-axial, or IVH without parenchymal hemorrhage and those with parenchymal isolated non-hemorrhagic injury were excluded. For all cases with definite or suspicion for fetal IPH based on the report review, fetal MRI was reviewed and those with no definitive parenchymal hemorrhage based on fetal single-shot fast spin echo (SSFSE) T2, T2* echo-planar imaging (EPI), T1 imaging, or postnatal MRI were excluded. All authors had equal access to the data set. This study was approved by the UCSF Institutional Review Board (Study #10–03219); individual participant consent was waived. The manuscript was prepared using the STROBE reporting guidelines with the RECORD extension.
Fetal and Postnatal Imaging
Fetal MRIs were reviewed by 2 pediatric neuroradiologists for the assessment of location of parenchymal hemorrhage (supra- vs infra-tentorial; frontal, temporal, parietal, and/or occipital lobe; and/or deep gray nuclei involvement). Extent of IPH was classified as focal, diffuse, or focal with associated diffuse non-hemorrhagic injury, and as unilateral or bilateral. Diffuse non-hemorrhagic injury was defined based on delayed sulcation for gestational age, diffuse parenchymal thinning (beyond the area of IPH, with or without associated ventriculomegaly [VM]), and/or diffuse abnormal parenchymal T2 signal (including abnormal multilayered pattern for gestational age). The presence of other abnormalities was assessed, including additional hemorrhage (GMH-IVH), VM (defined as mild to moderate = 10–15 mm or severe = > 15 mm), porencephaly, cortical malformation, and brainstem abnormality. Distinction of periventricular IPH from presumed venous occlusion (Papile grade 4) from other etiologies was challenging to determine on fetal MRI and hence Papile grades were not assigned. IPH size was assessed by measuring the largest diameter of parenchymal hemorrhage, hemorrhagic porencephalic cyst, or hemorrhagic schizencephalic defect. A ratio of IPH size to biparietal diameter was calculated. If multiple fetal MRIs were available, the largest IPH diameter and biparietal diameter were used. In cases of multiple hemorrhages, the largest IPH was used. Follow-up fetal MRI and/or postnatal brain MRI, when available, were categorized as demonstrating new findings or expected evolution of prior injury.
Clinical Data
Demographic data were collected from the internal database of patients with fetal MRI. Pre- and postnatal clinical data were extracted from available maternal and infant electronic medical records, including all investigations pertaining to the potential etiology of IPH. There was no standardized workup for etiology of fetal IPH across the long period of observation. Despite this, unless there was a clear alternative cause of IPH, most maternal-fetal dyads underwent infectious and coagulation evaluations at a minimum, and a subset underwent genetic testing. Etiology of IPH and clinical outcomes were classified by 2 pediatric neurologists who independently reviewed the records.
Etiology of IPH was grouped into 4 categories: (1) TTTS, (2) COL4A1/2 genetic variants, (3) other, and (4) unknown. The other category included those with a variety of maternal and/or fetal complications or comorbidities of hematologic, infectious, cardiac, cerebrovascular, or genetic nature. When the complication, intervention, or genetic variant had a known association with ICH or bleeding diatheses, etiology was classified as “definite/probable.” When documentation suggested a possible cause for the hemorrhage, but with either incomplete proof through testing, or only a proposed mechanism for association, etiology was marked as “possible.” Remaining cases were categorized as “unknown etiology,” with variable extent of testing performed. Given the long period of observation in this cohort, 2 epochs were defined as 1998 to 2009 and 2010 to 2022, divided equally across the 24-year time span, to compare differences in the identification of an etiology in the contemporary era with increased access to more advanced genetic testing.
Pregnancy outcomes included termination of pregnancy, radiofrequency ablation (RFA), spontaneous intrauterine fetal death (IUFD), and live birth. Neonatal outcomes included requirement for gastrostomy tube (GT) or death prior to hospital discharge. Neurologic outcomes were collected from the last available follow-up and were categorized based on presence of CP, epilepsy, ventriculoperitoneal (VP) shunt, global developmental delay (GDD), and/or intellectual disability (ID). GDD was defined as delays in 2 or more developmental domains impairing function. CP was graded using the Gross Motor Function Classification System (GMFCS); ambulatory CP (GMFCS I–III) was considered mild to moderate motor impairment, and non-ambulatory CP (GMFCS IV–V) was considered severe motor impairment. Individuals with severe motor disability, ID/GDD, or postnatal death were categorized as having severe neurologic impairment; all others were categorized as having mild to moderate neurologic impairment for statistical comparison.
Statistical Analysis
Descriptive statistics were used to summarize the demographics and tabulate the imaging findings, pregnancy, and postnatal outcomes. Imaging variables were compared among categories of etiology, combining both definite/probable and possible etiologies. Fisher's exact test was used for comparison of categorical variables; Mann Whitney U and Kruskal Wallis tests were used to compare median values for continuous variables. Significance was based on 2-tailed tests with an α of 0.05 and β of 0.20. All analyses used SPSS version 27.0.13
Results
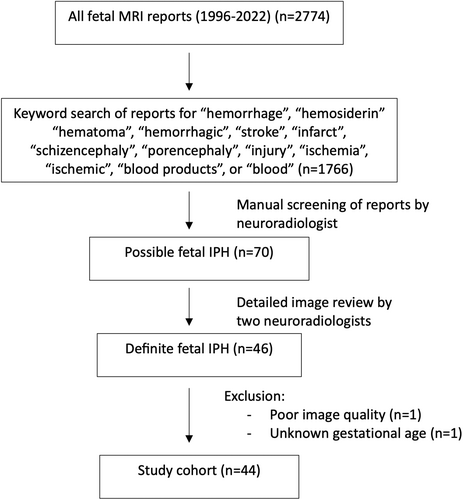
Of all fetal MRI reports (n = 2,774), 1,766 reports were identified by keyword search and reviewed, and 44 met inclusion criteria (Fig 1), including 34 singleton and 10 twin gestations (all monochorionic-diamniotic). Of all fetuses with prenatally identified sex (n = 39), 74% were male fetuses. Fetal MRI was prompted by brain abnormality identified on US in 29 of 44 cases, a brain abnormality plus known maternal/fetal complication in 4 of 44, and a brain abnormality and known twin gestation complication in 4 of 44. Seven fetuses had no identified brain abnormality on US but underwent fetal MRI for other indications (either known systemic disease or complications of twin pregnancy).
Fetal Imaging
Fetal MRI findings are summarized in Table 1, and examples of imaging findings are demonstrated in Figures 2 and 3. Median GA at the time of first fetal MRI was 24 weeks (interquartile range [IQR] = 22–28 weeks). IPH was supratentorial in 84%, and commonly focal (50%) or focal with diffuse non-hemorrhagic injury (43%). IPH was unilateral in 22 of 44 (left = 15/22 and right = 7/22), predominantly involved the frontal lobes (80%) and/or the parietal lobes (52%), and/or the deep gray nuclei (45%). GMH (75%), IVH (52%), and VM (75%), including 70% with severe VM, were common in this cohort. Median IPH diameter was 16.0 mm (IQR = 10.6–30.7 mm, n = 41). Median IPH/biparietal diameter was 0.3 (IQR = 0.2–0.6, n = 41). IPH diameter could not be measured in three cases due to the diffuse nature of IPH. Median IPH/biparietal diameter ratio was higher in those with IVH (0.5) compared to those without IVH (0.2, p = 0.002).
| TTTS N = 10 | COL4A1/2 N = 8 | Other fetal/maternal condition N = 15 | Unknown N = 11 | p value | |
|---|---|---|---|---|---|
| Gestational age at fetal MRI, weeks, median (IQR) | 23 (22–23) | 29 (22–33) | 25 (23–28) | 25 (22–29) | 0.245 |
| IPH Extent, n | 0.019 | ||||
| Focal IPH | 4 of 10 | 8 of 8 | 5 of 15 | 5 of 11 | |
| Diffuse IPH | 2 of 10 | 0 of 8 | 1 of 15 | 0 of 11 | |
| Focal IPH with diffuse injury | 4 of 10 | 0 of 8 | 9 of 15 | 6 of 11 | |
| IPH Location, n | 0.106 | ||||
| Supratentorial only | 10 of 10 | 8 of 8 | 9 of 15 | 10 of 11 | |
| Infratentorial only | 0 of 10 | 0 of 8 | 3 of 15 | 0 of 11 | |
| Supra- and infra-tentorial | 0 of 10 | 0 of 8 | 3 of 15 | 1 of 11 | |
| Bilateral IPH, n | 5 of 10 | 3 of 8 | 10 of 15 | 4 of 11 | 0.408 |
| IPH diameter, mm, median (IQR) | 17.9 (8.3–33.6) | 21.1 (13.0–34.7) | 13.4 (9.5–17.2) | 26.3 (15.5–30.9) | 0.217 |
| IPH/Biparietal diameter ratio, median (IQR) | 0.4 (0.2–0.8) | 0.3 (0.3–0.6) | 0.2 (0.2–0.4) | 0.4 (0.3–0.6) | 0.325 |
| Hemorrhagic porencephaly, n | 0 of 10 | 4 of 8 | 2 of 15 | 2 of 11 | 0.057 |
| Germinal matrix hemorrhage, n | 8 of 10 | 5 of 5a | 11 of 15 | 9 of 10a | 0.680 |
| Intraventricular hemorrhage, n | 6 of 10 | 4 of 6a | 3 of 15 | 10 of 10a | < 0.001 |
| Deep gray hemorrhage, n | 3 of 10 | 5 of 8 | 5 of 15 | 7 of 11 | 0.255 |
| Abnormal brainstem, n | 4 of 10 | 2 of 7a | 4 of 15 | 5 of 11 | 0.800 |
| Diffuse parenchymal thinning, n | 4 of 10 | 0 of 8 | 5 of 15 | 2 of 11 | 0.202 |
| Cortical malformation, n | 4 of 10 | 0 of 7a | 4 of 15 | 6 of 11 | 0.095 |
| Ventriculomegaly, n | 0.252 | ||||
| None (< 10 mm) | 4 of 10 | 1 of 8 | 5 of 15 | 1 of 11 | |
| 10–15 mm | 0 of 10 | 0 of 8 | 2 of 15 | 0 of 11 | |
| > 15 mm | 6 of 10 | 7 of 8 | 8 of 15 | 10 of 11 |
- Note: Bold p values represent p value < 0.05.
- a Variable denominator due to inability to categorize in certain cases based on fetal MRI quality or other limitation.
- IPH = intraparenchymal hemorrhage; IQR = interquartile range; MRI = magnetic resonance imaging; TTTS = twin-twin transfusion syndrome.
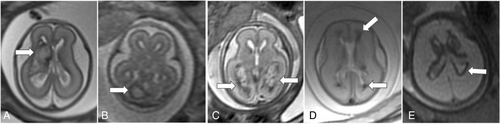
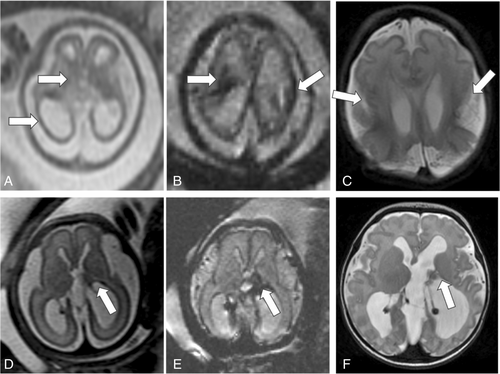
Follow-up fetal MRI was performed in 8 fetuses at median GA of 30 weeks (IQR = 25–31 weeks), occurring median 21 days (IQR = 14–29 days) from the initial MRI. The majority (n = 7/8) showed expected evolution of IPH; one fetus had a new finding of polymicrogyria overlying the hemorrhage.
Etiology
A definite or possible etiology was identified in 85% (33/39) of patients who underwent a workup and in 75% (33/44) of all cases (Table 2). The most common etiologies of fetal IPH were TTTS (n = 10), COL4A1/2 variants (n = 8), and intrauterine transfusion (IUT; n = 5). After excluding twin pregnancies, those with IUT, congenital infections, and those that deferred any testing, the yield of genetic testing was 9 of 23 (39%). Etiology of IPH was associated with extent of IPH (p = 0.019) and presence of IVH (p < 0.001), which is further discussed by etiology below.
| Definite/probable (n = 30) |
| TTTS (n = 10) |
| COL4A1/2 mutation (n = 8) |
| Other fetal/maternal hematologic, infectious, cardio-/cerebro-vascular, or other genetic abnormalities (n = 12) |
| IUT (n = 5) |
|
|
|
| Platelet abnormality (n = 3) |
|
|
|
| Other comorbidity (n = 3) |
|
|
|
| Congenital infection (n = 1) |
|
| Possible (n = 3) |
|
|
|
| Unknown (n = 11) |
|
|
- CMPL+ = congenital myeloproliferative disorder positive; CMV = cytomegalovirus; CPAM = congenital pulmonary airway malformation; IPH = intraparenchymal hemorrhage; IUT = intrauterine transfusion.
The 10 fetuses with IPH related to TTTS had no significant differences in pattern of IPH compared to the non-TTTS group. All had supratentorial and bilateral IPH; 6 of 10 had concomitant IVH. In 7 of 10 cases, placental laser ablation therapy was performed at median 12 days (range = 2–36 days) prior to fetal MRI. In 7 of 10 cases, the affected twin was the TTTS recipient. In 3 of 10 cases, there was demise of the co-twin prior to fetal MRI (only 1 of these 3 had undergone laser ablation therapy). In fetuses with TTTS and hydrops, diffuse periventricular T2 hypointensity appeared to be reversible, not associated with surrounding edema, and did not have postnatal imaging sequelae, ultimately thought to reflect engorgement of the medullary veins related to hydropic state.
Among the 8 fetuses with IPH related to COL4A1/2 variants, all had focal IPH, compared to the non-COL4A1/2 group, in which only 14 of 36 had focal IPH (p = 0.004). Half of the fetuses with COL4A1/2 had hemorrhagic porencephaly, compared to 4 of 36 in the non-COL4A1/2 group (p = 0.026). This cohort with COL4A1/2 variants is described in further detail in a previously published study.14
Five fetuses had IPH associated with IUT for severe fetal anemia; 2 had additional potential risk factors for IPH, including one with parvovirus and one with a chromosomal abnormality. Four had one IUT prior to identified IPH and 1 had 2 IUTs prior to identified IPH. Median GA at IUT prior to IPH identification was 24 weeks (range = 18–28 weeks); fetal MRI occurred on median day 5 after IUT (range = 3–18 days). Infratentorial IPH occurred in 5 of 5 cases due to IUT (3/5 with both supra- and infra-tentorial IPH) compared to only 2 of 39 with infratentorial IPH (1 with both supra- and infra-tentorial IPH) in the non-IUT group (p < 0.0001).
In addition to the 5 fetuses with IUT, 10 fetuses had IPH related to a variety of other causes (see Table 2). IVH was less frequently observed in this group (2/10) compared to the remainder of the cohort (21/34, p = 0.031). Otherwise, there were no other distinct imaging characteristics in this heterogeneous subset.
Eleven fetuses had unknown etiology of IPH despite variable degrees of evaluation. Six underwent some degree of a workup, although only one had known COL4A1/2 testing (which was negative). Five were lost to follow-up or had no further testing.
Comparing cases from the early versus late epochs, there was no significant difference in the likelihood of identifying an etiology for the IPH in the early earlier epoch (n = 7/11) compared to the later epoch (n = 26/33, p = 0.425), although all cases of COL4A1/2 were identified after 2010. Among cases with unknown etiology, only 1 of 4 underwent some degree of a workup in the early epoch, whereas 5 of 7 underwent some degree of a workup (usually neonatal alloimmune thrombocytopenia [NAIT] testing and/or single nucleotide polymorphism [SNP] array) in the later epoch.
Pregnancy and Perinatal Outcomes
Within the cohort, 43 of 44 fetuses had known pregnancy outcome; of these, 22 of 43 were live born, 3 of 43 had IUFD, 2 of 43 underwent RFA, and 16 of 43 pregnancies were terminated (Supplementary Table S1). Median GA at live birth was 38 weeks (IQR = 35–39 weeks) and 17 of 22 (77%) were male fetuses. Five of 22 fetuses were born preterm at < 37 weeks GA, and 2 died during the birth hospitalization. In cases of pregnancy termination, median IPH/biparietal diameter ratio was larger compared to those who were live born or had spontaneous IUFD (p = 0.002). Termination of pregnancy was less common when VM was absent (p = 0.003). The number of live births and terminations were not significantly different between the 2 epochs.
Postnatal Imaging
Postnatal brain MRI was variably performed (n = 13/22 with live births), most commonly in the neonatal period (median = 2 days of life). New findings were observed in 6 of 13 patients, including increasing VM or hydrocephalus (n = 2), new hemorrhage (n = 2), polymicrogyria (n = 1), and diffuse white matter injury (n = 1).
Childhood Outcomes
Of the 20 liveborn infants that survived the perinatal period, 1 died prior to 12 months of age. No clinical data were available for 3 liveborn infants, and an additional 3 had no clinical data available after 12 months of age. In total, 13 of 19 liveborn, surviving infants had available follow-up data beyond 12 months of age at median age 7 years (IQR = 2–10 years; Supplementary Table S2). Among those with follow-up beyond 12 months, 12 of 13 children have CP, including 3 of 12 with severe motor impairment and 9 of 12 with mild to moderate motor impairment. Six have ID/GDD. Five children have epilepsy. Two children (both with CP GMFCS V) required a GT placed during the neonatal period. Two children (one with CP GMFCS I, and 1 without CP) required VP shunt placement.
Imaging Findings, Etiology, and Clinical Outcomes
Of all liveborn fetuses with any available follow-up data (n = 19), etiology of IPH was significantly associated with neurodevelopmental outcome (Table 3). Those with other etiology including IUT had higher risk of severe neurologic outcome or death (p = 0.022). Fetal IVH and severe VM were more common in children with mild to moderate neurologic impairment compared to children with severe impairment or death. Diffuse IPH was associated with need for GT (2/2 with diffuse hemorrhage vs. 0/11 with focal hemorrhage, p = 0.013).
| Mild to moderate neurologic impairment (n = 9) | Severe neurologic impairment/death (n = 10) | p value | |
|---|---|---|---|
| Gestational age at fetal MRI, weeks, median (IQR) | 26 (23–30) | 27 (25–29) | 0.905 |
| IPH Extent, n | 0.221 | ||
| Focal IPH | 7 of 9 | 4 of 10 | |
| Focal IPH and diffuse injury | 2 of 9 | 4 of 10 | |
| Diffuse IPH | 0 of 9 | 2 of 10 | |
| IPH Location, n | 1.00 | ||
| Supratentorial only | 8 of 9 | 8 of 10 | |
| Infratentorial only | 1 of 9 | 1 of 10 | |
| Supra- and Infra-tentorial | 0 of 9 | 1 of 10 | |
| Bilateral IPH, n | 2 of 9 | 7 of 10 | 0.070 |
| IPH diameter (mm), median (IQR) | 15.5 (11.4–16.6) | 9.7 (7.4–17.1) | 0.236 |
| IPH/Biparietal diameter ratio, median (IQR) | 0.2 (0.2–0.3) | 0.2 (0.1–0.2) | 0.690 |
| Hemorrhagic porencephaly, n | 2 of 9 | 2 of 10 | 1.00 |
| Germinal matrix hemorrhage, n | 6 of 7 | 6 of 9 | 0.585 |
| Intraventricular hemorrhage, n | 6 of 7 | 1 of 10 | 0.004 |
| Deep gray hemorrhage, n | 5 of 9 | 2 of 10 | 0.170 |
| Abnormal brainstem, n | 0 of 9 | 2 of 10 | 0.092 |
| Diffuse parenchymal thinning, n | 1 of 9 | 5 of 10 | 0.141 |
| Cortical malformation, n | 1 of 8 | 3 of 10 | 0.588 |
| Ventriculomegaly, n | 0.057 | ||
| None | 1 of 9 | 6 of 10 | |
| 10 to 15 mm | 0 of 9 | 0 of 10 | |
| > 15 mm | 8 of 9 | 4 of 10 | |
| Etiology, n | 0.022 | ||
| TTTS | 1 of 9 | 2 of 10 | |
| COL4A1/2 | 4 of 9 | 1 of 10 | |
| Other fetal/maternal comorbidity | 1 of 9 | 7 of 10 | |
| Unknown | 3 of 9 | 0 of 10 |
- Note: Bold p values represent p value < 0.05.
- IPH = intraparenchymal hemorrhage; IQR = interquartile range; MRI = magnetic resonance imaging; TTTS = twin-twin transfusion syndrome.
Discussion
This largest-to-date study of fetal IPH observed preliminary associations among etiology, fetal MR imaging findings, and postnatal outcomes. Fetal IPH was most commonly supratentorial and focal, or focal with superimposed diffuse non-hemorrhagic brain injury. Most cases of fetal IPH had associated GMH and/or IVH. An etiology of fetal IPH was identified in most cases, and TTTS, COL4A1/2 variants, and IUT were the most common causes of fetal IPH in our cohort. Features of IPH on fetal MRI may provide clues to the underlying cause, such as focal IPH with hemorrhagic porencephaly in COL4A1/2 variants, and infratentorial IPH in IUT. Etiology and fetal imaging findings were associated with neurologic outcome in this cohort, although larger studies with postnatal follow-up are needed.
Consistent with demographic observations in other perinatal stroke populations, fetal IPH was more common in male patients in this cohort. Perinatal stroke occurs more commonly in male patients,15, 16 for unknown reasons, and male predominance in fetal ICH has previously been suggested,17 although conclusions are limited by inconsistent reporting of sex in fetal imaging cohorts. Live birth was observed in half of the cohort, lower than in meta-analyses of fetal ICH that include a wider range of ICH types, including milder cases such as isolated low-grade GMH-IVH.4, 9 The rate of live birth after fetal IPH is likely influenced by multiple factors, including GA at time of imaging, legal access to termination of pregnancy, and patient preferences and values. In our cohort, VM and ratio of IPH diameter to biparietal diameter were significantly associated with pregnancy outcome. One possible explanation is that larger IPH prompted counseling about more severe potential outcomes, or expectant parents may heavily weigh the presence of VM or the size of hemorrhage in their decision-making process, possibly resulting in higher rates of termination compared to fetuses with smaller IPH. Assessing the numerous factors that may influence decision making around pregnancy continuation in fetal IPH is beyond the scope of this paper. Access to multidisciplinary care teams, including maternal-fetal medicine specialists, fetal neurologists, and genetic counselors, is essential for providing optimal care for the fetus and families in these clinical scenarios.18-20
Expanding on the MERIDIAN study,21 which did not report on ICH, we observed that fetal MRI detected more brain abnormalities than fetal US in our cohort of IPH. New findings were rare in those with a second fetal MRI, but new findings were observed in half that underwent postnatal brain MRI, supporting the need to obtain postnatal MRI to detect additional complications related to IPH, such as recurrent hemorrhage, progression of VM, polymicrogyria, or WM injury. Our cohort demonstrates that fetal IPH can present in a variety of locations, but primarily affects the supratentorial brain (most commonly frontal and parietal lobes). This observation is similar to the predominant supratentorial location of hemorrhage in a recent fetal ICH cohort,22 although that cohort grouped both intra- and extra-axial hemorrhage in analyses.
The salient aspects of fetal IPH pattern were location and extent of associated injury. In our cohort, infratentorial IPH was almost exclusively observed in the fetuses who underwent IUT, similar to previous case reports of fetal IUT and cerebellar hemorrhage.23-25 The cause of cerebellar-predominant hemorrhage after IUT is not well understood, but may relate to timing of the procedure and selective vulnerability of the cerebellar germinal matrix.26 Additionally, we observed that fetal IPH was almost always focal but variably associated with diffuse non-hemorrhagic injury. Fetuses with COL4A1/2 variants had exclusively focal injury whereas those with other etiologies of IPH had variable degrees of parenchymal injury, suggesting IPH etiology influences extent of associated non-hemorrhagic injury. Timing of IPH and the time interval between IPH and fetal MRI likely affect observed injury on fetal MRI. A larger cohort is needed to further untangle multiple factors related to patterns of parenchymal injury in fetal IPH.
Etiology of fetal IPH, compared to other types of intracranial hemorrhage, is not well understood. In our cohort, an etiology was identified in 85% (33/39) of patients who underwent workup, and etiology demonstrated an association with severity of neurologic impairment. In a recent meta-analysis of all types of fetal ICH (including both intra- and extra-axial hemorrhage), an etiology was identified in only 37%.4 Previously reported common etiologies of fetal ICH are FNAIT,27, 28 genetic conditions including COL4A1/2 variants,29, 30 infection,4 and other pregnancy complications.9 Interestingly, FNAIT was only identified in 1 of 33 and infection only in 2 of 33 fetuses in our cohort, suggesting they may be less common causes of fetal IPH than previously suspected. Alternatively, these cases may not have undergone fetal MRI and therefore would not have been identified, as ICH due to FNAIT generally occurs late in the second trimester.28 Geographic location of our cohort may have also influenced the prevalence of congenital infections.31 The high rate of an identifiable etiology in this study is likely due to a variety of factors, including access to testing and a relatively homogenous cohort with exclusion of isolated low-grade IVH which typically does not have an identifiable underlying cause.9 As low-grade GMH-IVH is thought to have a generally favorable outcome, and access to extensive genetic testing has been limited until more recently, prior studies may have under-recognized an underlying etiology even in low grade GMH-IVH. Higher grade GMH-IVH, including those with IPH (as included in this study), may be more likely to have an identifiable etiology, as suggested in this study and others.32 Improved access to fetal MRI and genetic testing will continue to expand our understanding of fetal IPH with and without concomitant IVH.
TTTS was the etiology of IPH in all twin gestations in our cohort. TTTS and associated complications such as co-twin demise, and interventions such as laser ablation are known risk factors of fetal brain injury.33-35 Although cerebral abnormalities are seen in 8% to 23% fetuses with TTTS,36, 37 parenchymal hemorrhagic injury is rare (< 1%) in fetuses with TTTS.38 Low flow states are thought to result in ischemic lesions, whereas high flow states result in hemorrhagic lesions but there is no distinct pattern of injury in donors compared to recipients.37
Genetic variants were a common etiology of IPH in singleton pregnancies, accounting for 9 of 23 (39%) of all singleton fetuses without congenital infection or IUT who underwent some degree of genetic testing (microarray +/− whole exome sequencing). Of these, COL4A1/2 variants accounted for 8 of 23 (35%). Several studies have found similar prevalence of COL4A1/2 mutations in fetal ICH.29, 30 Collagenopathies have also been identified as a potential cause of PVHI in preterm and full-term neonates.32 Given variable clinical practice and access to genetic testing across the observation period, the overall prevalence of pathogenic variants in this cohort may be underestimated. Congenital amegakaryocytic thrombocytopenia (caused primarily by mutations in the MPL gene) and other genetic hematologic conditions may be under-recognized causes of perinatal hemorrhagic stroke.39 Our data suggests expanded whole exome/genome testing should be offered in all cases of fetal IPH where there is not a readily identified cause, such as complicated twin pregnancy, IUT, or infection, particularly in those with focal IPH without diffuse injury, hemorrhagic porencephaly, or those with abnormal deep gray nuclei. Given that identification of genetic variants also has implications for management of systemic comorbidities, consultation with a geneticist, parental testing, and counseling for future pregnancies may be indicated.
There is a paucity of literature on long-term outcomes after fetal IPH, with or without GMH-IVH, compared to what is known about isolated fetal IVH, although most studies on fetal IVH also include a subset with PVHI, which has a parenchymal component. Meta-analyses on fetal ICH have included fetuses with a wide spectrum of ICH type and severity, making it challenging to specifically extrapolate data on outcomes to fetuses with parenchymal hemorrhage. In studies including fetal PVHI or IPH, observed rates of moderate to severe neurologic impairment are high.4, 10, 11 A recent meta-analysis on GMH-IVH (including 133/240, 55% with fetal MRI), observed that more than 50% of surviving fetuses with PVHI required VP shunt placement; over 75% had motor impairment, over half had developmental delay, and one quarter had epilepsy.9 Those with clinical follow-up in our cohort had lower rates of VP shunt placement but otherwise similar rates of long-term neurologic sequelae. In liveborn individuals in our cohort, presence of IVH and/or severe VM on fetal MRI was associated with lower likelihood of severe neurologic impairment or death, and we hypothesize this association may be mediated by etiology of IPH or censoring due to pregnancy termination. Aside from diffuse IPH being associated with requirement for GT placement, no other fetal imaging characteristics were associated with severity of neurologic outcome, although assessment is limited by the small number with long term follow-up and larger studies are warranted.
Our study addresses a key gap in the available literature on fetal ICH, specifically fetal IPH, and in fetal neurology. Fetal neurology is a new emerging subspecialty in which there are no standardized practice guidelines, and there remains a very limited literature upon which to base our counseling and care. As shown in a recent practice survey of pediatric neurologists that provide fetal counseling,20 our community endorses a need for guidelines, which can only be developed through building the knowledge and evidence base in this uniquely complex clinical population. Findings from our largest-to-date IPH cohort have immediate implications for clinical care, including prenatal counseling and etiologic evaluation, and importantly to guide future research.
There are several limitations to our study. This was a retrospective study, and over the duration of data collection, imaging techniques, clinical management, and prenatal counseling have evolved both at our institution and worldwide. Imaging distinction of parenchymal hemorrhage from calcification is challenging on fetal MRI even in the presence of T2, T1, and T2* sequences. In some cases, only SSFSE T2 sequences were acquired, and postnatal imaging was used for confirmation of prior hemorrhage, when available. Determination of IPH etiology was also limited by heterogenous testing practices and retrospective chart review. In particular, genetic testing has evolved substantially over the past years, from karyotype testing to whole exome/genome sequencing. Genetic testing was not universally performed in this cohort, even in recent years, due to variable clinical practices and patient preference, possibly underestimating the prevalence of genetic abnormalities. Future prospective studies of fetal IPH and GMH-IVH will benefit from systematic testing for determination of etiology. Termination of pregnancy, lack of identification of etiology of IPH despite extensive testing, and patient loss-to-follow-up limit the sample size in this cohort for assessment of associations of imaging findings with etiology and outcomes.
Conclusions
Fetal IPH is a rare diagnosis that is associated with substantial morbidity and mortality. Fetal MRI allows for characterization of parenchymal injury and associated complications of fetal IPH. Twin pregnancy-related complications and genetic conditions including COL4A1/2 variants are the most common causes of fetal IPH with or without GMH-IVH in this cohort. Cerebral palsy is very common, but most children in the cohort with follow-up are ambulatory. Characteristics of IPH on fetal MRI demonstrate preliminary association with etiology and outcomes, and further studies of a larger, multicenter cohort of fetal IPH and GMH-IVH are warranted.
Acknowledgments
The authors would like to acknowledge the following funding sources: Brain Vascular Malformation Consortium (Rachel Vassar), ASNR Scholar Grant (Elizabeth George), RSNA Scholar Grant (Elizabeth George), and the UCSF Department of Radiology RIDR Program (Andrew Mogga).
Author Contributions
R.V., E.G., O.G., and D.G. contributed to the conception and design of the study. R.V., E.G., A.M., Y.L., M.N., O.G., and D.G. contributed to the acquisition and analysis of data. R.V., E.G., M.N., O.G., and D.G. contributed to drafting a significant portion of the manuscript or figures.
Potential Conflicts of Interest
Nothing to report.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.